Computational Immunology, Utrecht, December 7th, 2022
T-cell collectives in silico
Emerging complexity in
T-cell repertoires and
T-cell migration
Inge Wortel
Computational Immunology group,
Radboud University Nijmegen, NL
inge.wortel@ru.nl![]()
@inge_wortel![]()
About me
Background:
 |
 |
 |
| 2011-2014 BSc Chemistry @Radboud Uni |
2011-2014 BSc Molecular Life Sciences @Radboud Uni |
2014-2016 MSc Molecular Mechanisms of Disease @Radboud Uni |
About me
 |
2016-2021: PhD Computational Immunology Computational modelling of T-cell collectives, computational immunology group @Radboudumc 2021-2022: Postdoc Computational Immunology computational immunology group (moved to Data Science @Radboud Uni) Now: Starting my own group Combining machine learning and simulation to understand biological complexity (@Data Science, Radboud Uni) |
T-cell collectives: a whole that is more than the sum of its parts?
Our T-cell collective:
- contains millions of T cells
- these T cells interact
$\rightarrow$ "complex" behaviors can emerge!
...so how can we know what T cells will do?
Modelling the T-cell collective
Two topics:
- T-cell migration (now)
- the T-cell repertoire (later)
Computational Immunology, Utrecht, December 7th, 2022
Part I
Could T cells form traffic jams?
The biological problem.
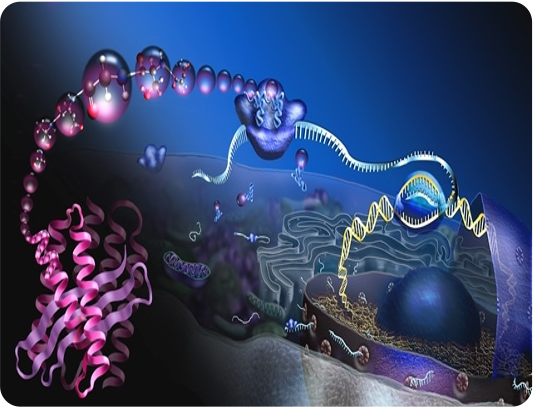
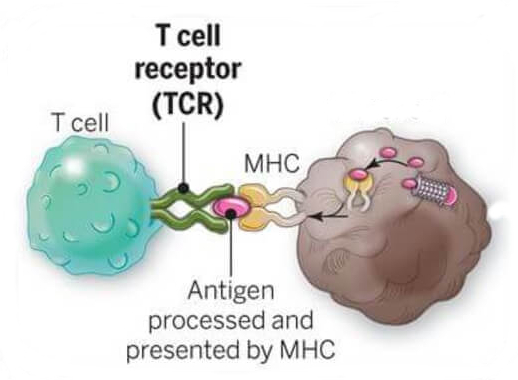
T cells recognize compromised cells specifically.
Adapted from National Cancer Institute (NIH)
- Adaptive immune system
- Recognize pathogen-infected or cancerous cells
- T-cell receptor (TCR) recognizes short peptides on MHC
- Compromised (infected/cancerous) cells display different peptides than healthy cells do
- Specific because each T cell's receptor recognises specific peptides
T cells search for anomalies in lymph nodes.
What are we missing?
Only one in a million T cells can detect any given new pathogenic signal (peptide).
T cells must search for these rare targets that can activate them.
They do this in central "meeting hubs" called lymph nodes.
A. Peixoto, Harvard Medical School
Hidden figures.
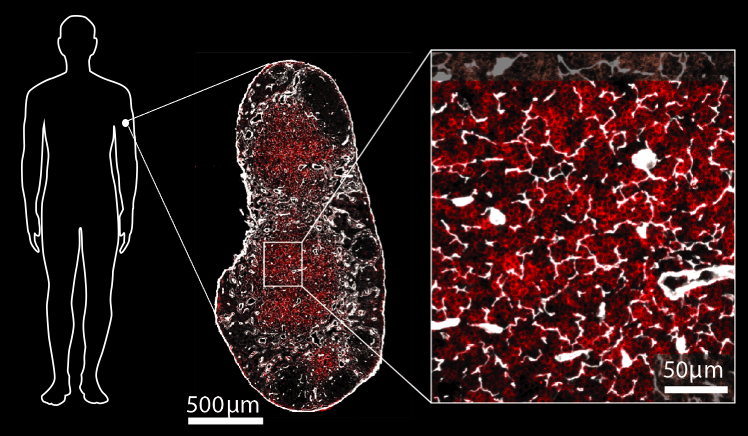
Image: Connie Shen & Judith Mandl.
The question: why no traffic jams?

|
? |
How do T cells respond to complex and crowded environments, and does their smooth traffic flow ever break down?
mechanistic
modelling.

"What I cannot create, I do not understand."
— Richard Feynman
"Creating" real and simulated T-cell crowds.
Put T cells in controlled environments, inspired by the physics field of crowd dynamics.
- In silico: computational model
- In vitro: controlled environment in the lab
Can we "build a crowd" — i.e., can our model predict what real T cells will do in vitro?
Data: time-lapse imaging of moving cells. Predictions: what will the crowd do?
The circle of life mechanistic modelling.


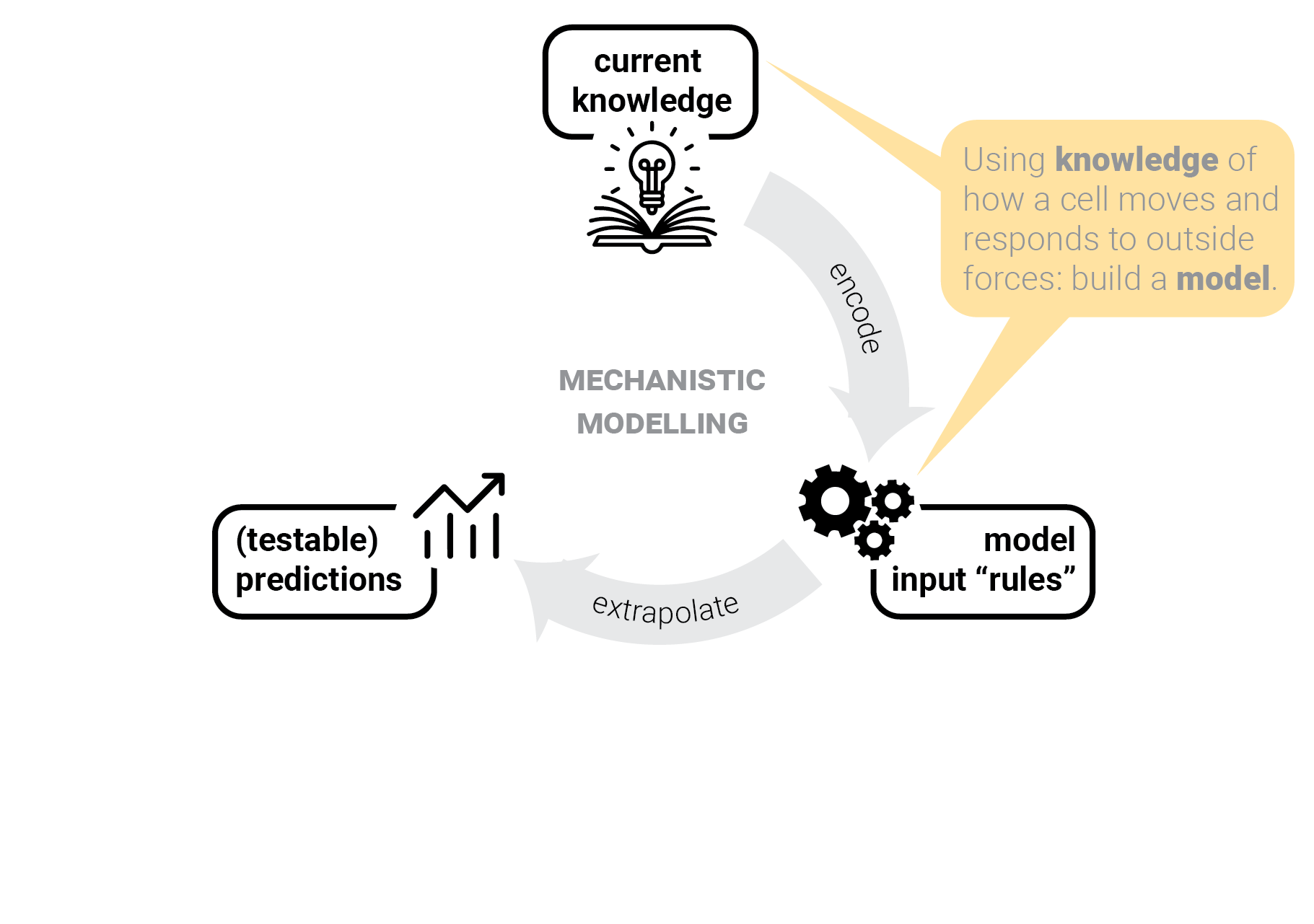
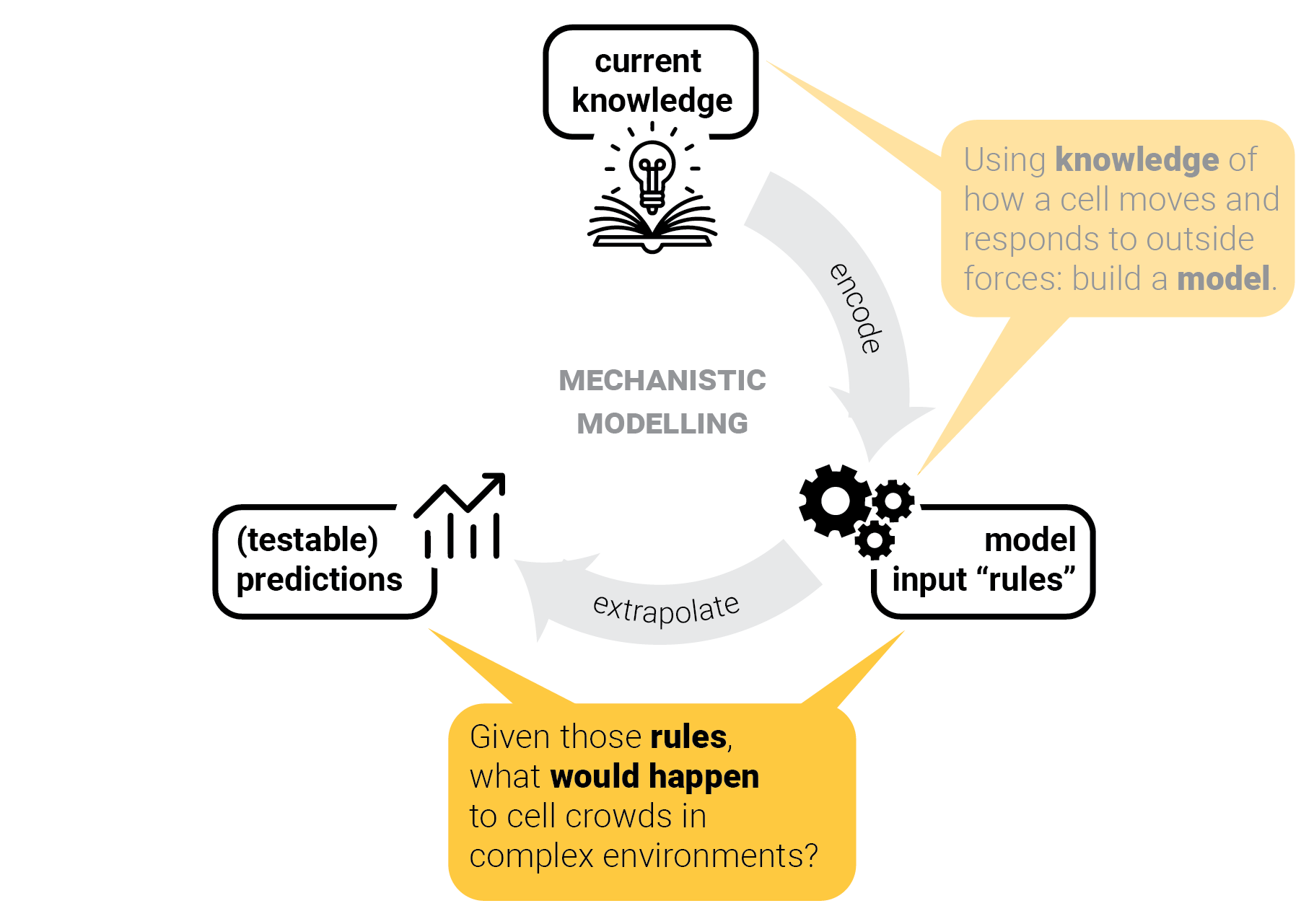
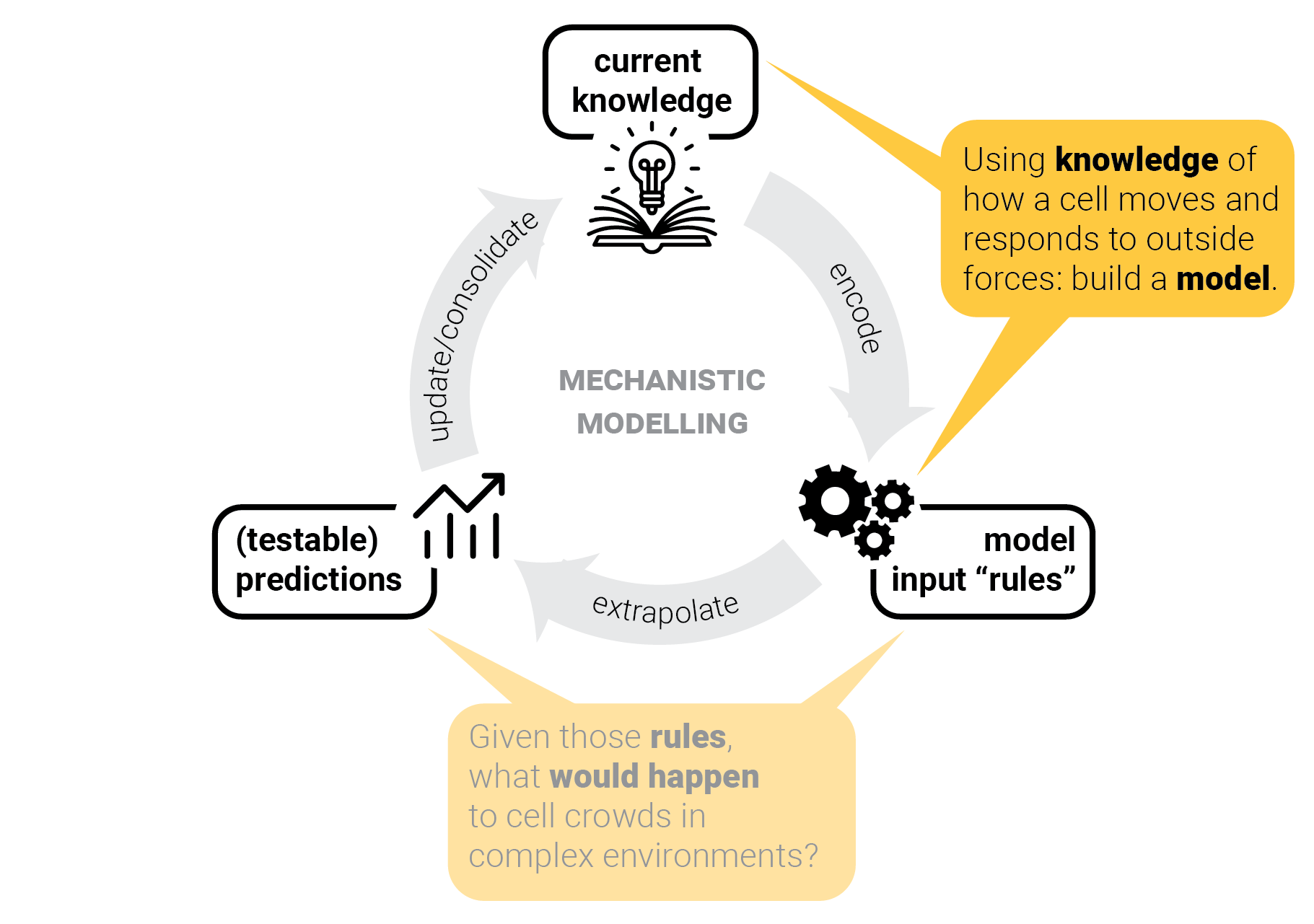

T-cells in one lane traffic.
Step 1: gather input knowledge.

Step 1: gather input knowledge.

Step 2: encode into a model.
Option 1: Detailed model
Explicitly encode every molecule and resulting force.
+ highly interpretable!
+ emergent behavior.
— too expensive to model crowds.
Step 2: encode into a model.
Option 2: Phenomenological — Cellular Potts Model (CPM)1
Pixels belong to cells, which
move by copying pixels:
Copy success chance (Pcopy) is higher when it helps the cell:
Powered by Artistoo.net
$P_\text{copy} = \begin{cases} e^{-\Delta H/T} & \Delta H \gt 0\\ 1 & \Delta H \leq 0 \end{cases}$
$\rightarrow$ Cells have shapes and interact naturally through volume exclusion (each pixel can only belong to one cell at a time). Crowd behavior still emerges.
1Graner and Glazier (1992). doi:10.1103/PhysRevLett.69.2013
Step 2: encode into a model.
Powered by Artistoo.net
Cells move if we add positive feedback on protrusive activity ($\approx$ actin polymerization)1:
| Parameters: | ||
| λact | $\approx$ | protrusive force |
| maxact | $\approx$ | polymerized actin lifetime |
$\rightarrow$ realistic cell shape and motility 1,2.
1Niculescu et al. (2015). doi:10.1371/journal.pcbi.1004280
2Wortel et al. (2021). doi:10.1016/j.bpj.2021.04.036
Step 3: predict crowd behavior.
A cornerstone scenario in crowding physics: one-lane traffic.

1John et al. (2009). doi:10.1103/PhysRevLett.102.108001
2Seyfried et al. (2005). doi:10.1088/1742-5468/2005/10/p10002
What do you think?
T cells are like:
A. Humans
B. Ants
Step 3: predict crowd behavior.
What do T cells do? Put single (CPM) cells together in constrained channels and predict crowd behavior:
Qualitatively: cells rapidly align into "trains" to keep moving.
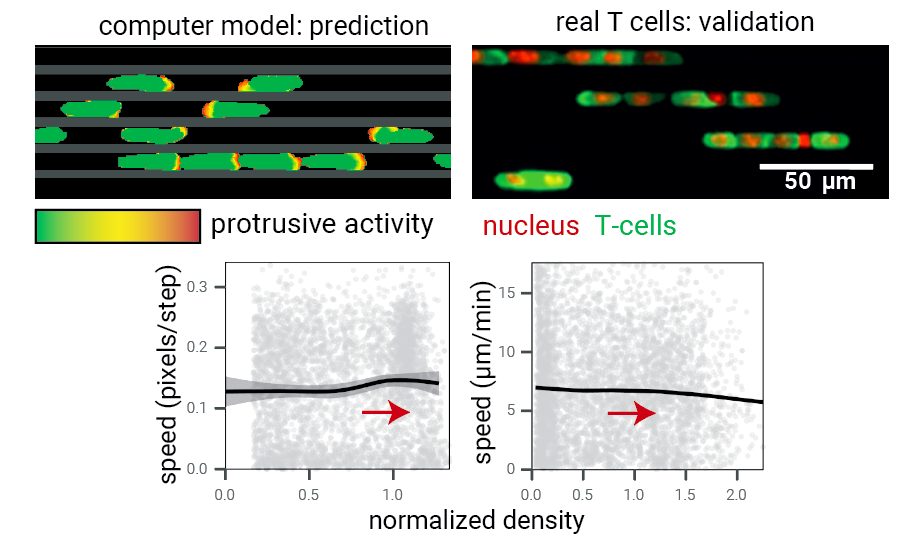
Step 4: test model predictions.
What about real T cells? Again: train formation!
Data: Jérémy Postat and Judith Mandl.
Step 4: test model predictions.
Quantitatively: the fundamental diagram in both cases is flat.
Step 5: consolidate model — and repeat.
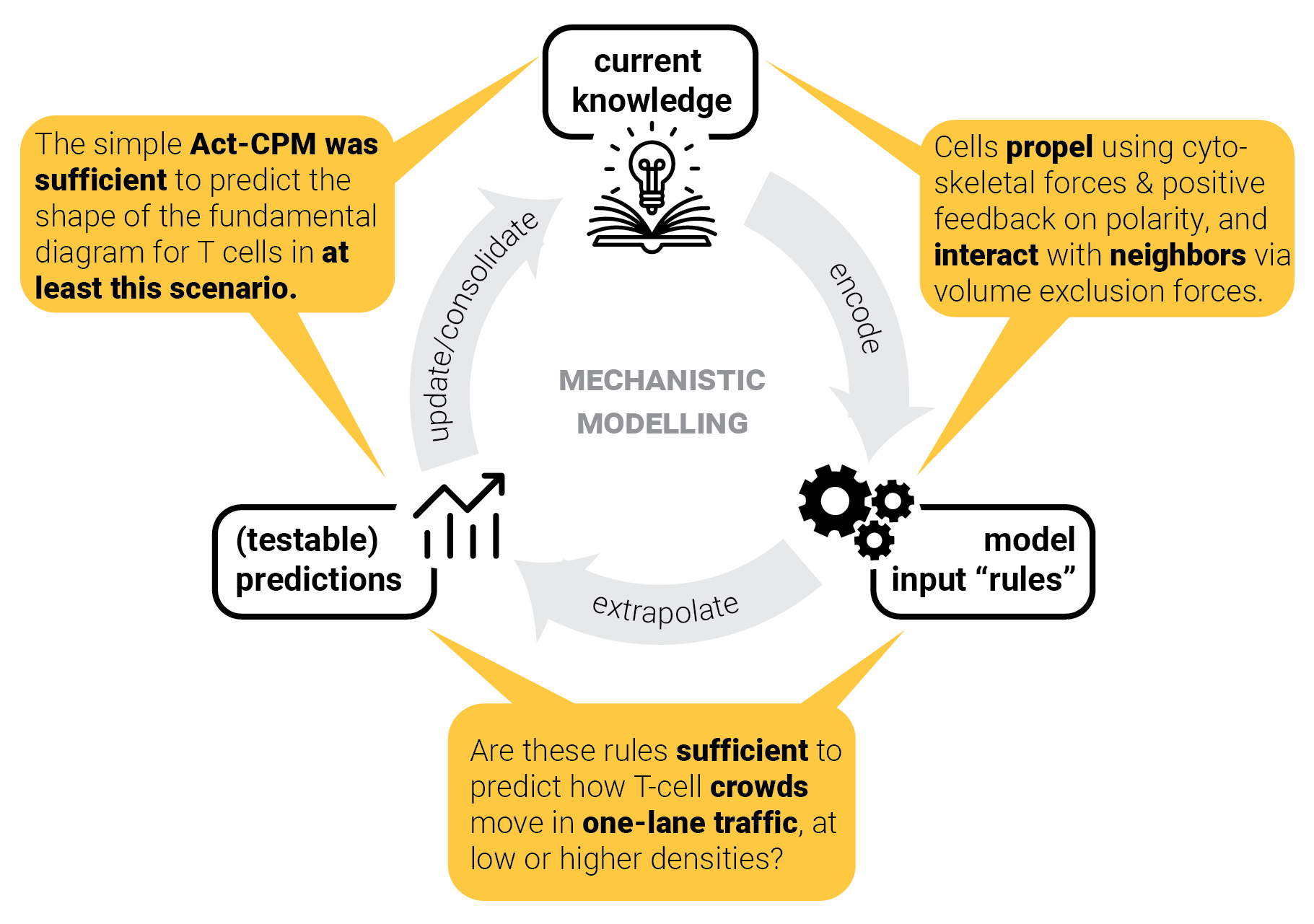
Model consolidation != proof.
Can we predict crowd behavior in other scenarios as well?
Step 5: consolidate model — and repeat.
Pedestrian crowds can form jamming arches near an exit. This scenario is well-studied because of crowd disasters, such as at the Love Parade (Berlin, 2010).
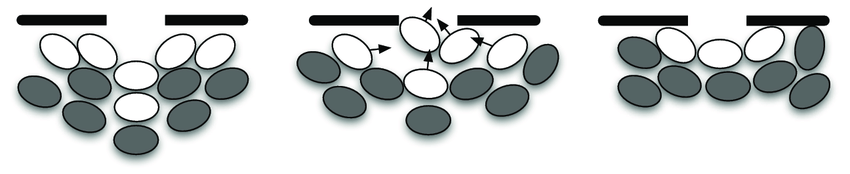
$\rightarrow$ What about T cells?
Step 5: consolidate model — and repeat.
Simulated T cells can indeed form jamming arches:
Work in progress, but see: Wortel (2021). https://repository.ubn.ru.nl/handle/2066/236680.
Step 5: consolidate model — and repeat.
Over time, the system alternates between a "jammed" and a "flowing" state:

Faster is slower?
By varying model parameters, we can see what happens when the escape process is more competitive (i.e.: cells pulled more strongly to the right):

Faster is slower?
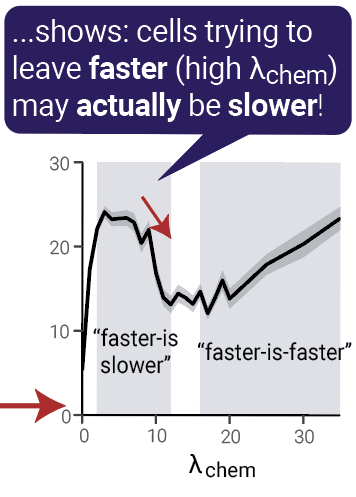
We call this the "faster-is-slower" effect:
- Cells that try to escape faster are actually slower because they block the exit
- Known from other systems (e.g. pedestrians)
- Stops when forces become high enough
But: "to be continued" — more validation is needed:
- What if cells are more deformable?
- What happens in vitro.
Summary: on the existence of T-cell traffic jams
T cells are inherently quite jam-resilient:
- They self-align to avoid jamming in one-lane traffic
- resulting in a "flat" fundamental diagram like that of ants.
...but jamming may occur in challenging environments:
- (temporary) jamming arches form during competitive escape
- T cells might even exhibit the "faster-is-slower" effect, but this awaits validation.
Work of Shabaz Sultan
Read more
About the model:
- https://artistoo.net/explorables/Explorable-CPM.html
- https://artistoo.net/explorables/Explorable-ActModel.html
...Or try out the simulations yourself:
Acknowledgments
 |
 |
 |
 |
 |
|
| Jérémy Postat | Connie Shen | Judith Mandl | Shabaz Sultan | Johannes Textor | |
| Mandl lab McGill University, Montréal, Canada |
Computational immunology group Radboud University, the Netherlands |
||||
| computational-immunology.org | |||||
 |
 |
||||