Group meeting Mandl lab February 4th, 2020
The fast and the curious:
Can T cells tune speed and turning behavior
to optimize their search for antigen?
Inge Wortel
inge.wortel@radboudumc.nl
Department of Tumor Immunology, Radboudumc



Search strategies: the exploration-exploitation dilemma
How can I find my keys asap? Two things matter:
1. How fast do I move? (speed)

| Faster : | cover more area, |
| but less time to look |
2. How long until I turn? (persistence)

| Short time : | more thorough |
| but get less far |
Images adapted from: Benichou et al. Review of Modern Physics, 2011.
Searching in immunology
|
T cells in uninfected LN A. Peixoto, Harvard Medical School |
Neutrophils in an infected lung
Chtanova et al, Immunity 2008. |
How T cells search
T cells show highly different migratory behavior depending on context:
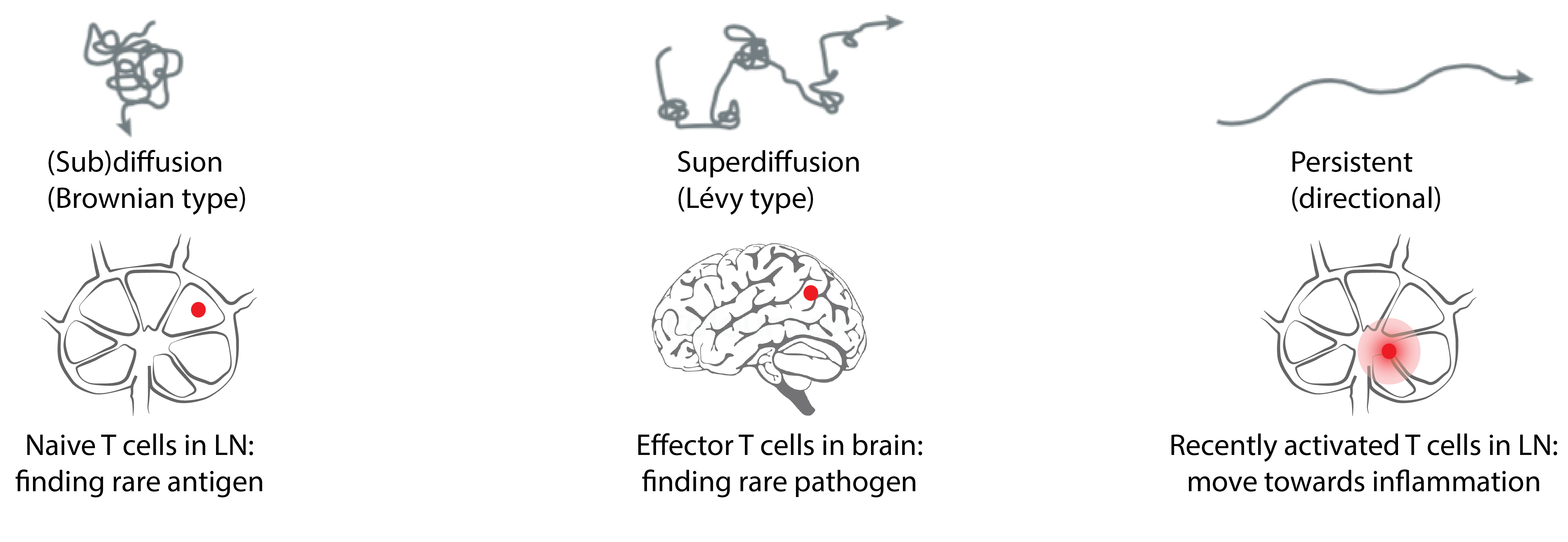
$\rightarrow$ Does this reflect different "optimal" search strategies?
Image adapted from: Krummel et al. Nature Reviews Immunology, 2016.
Investigating T cell search: random walk models

A cell takes a series of steps
to find targets. We can tune:
- μ (step length)
- v (step speed)
How T cells search
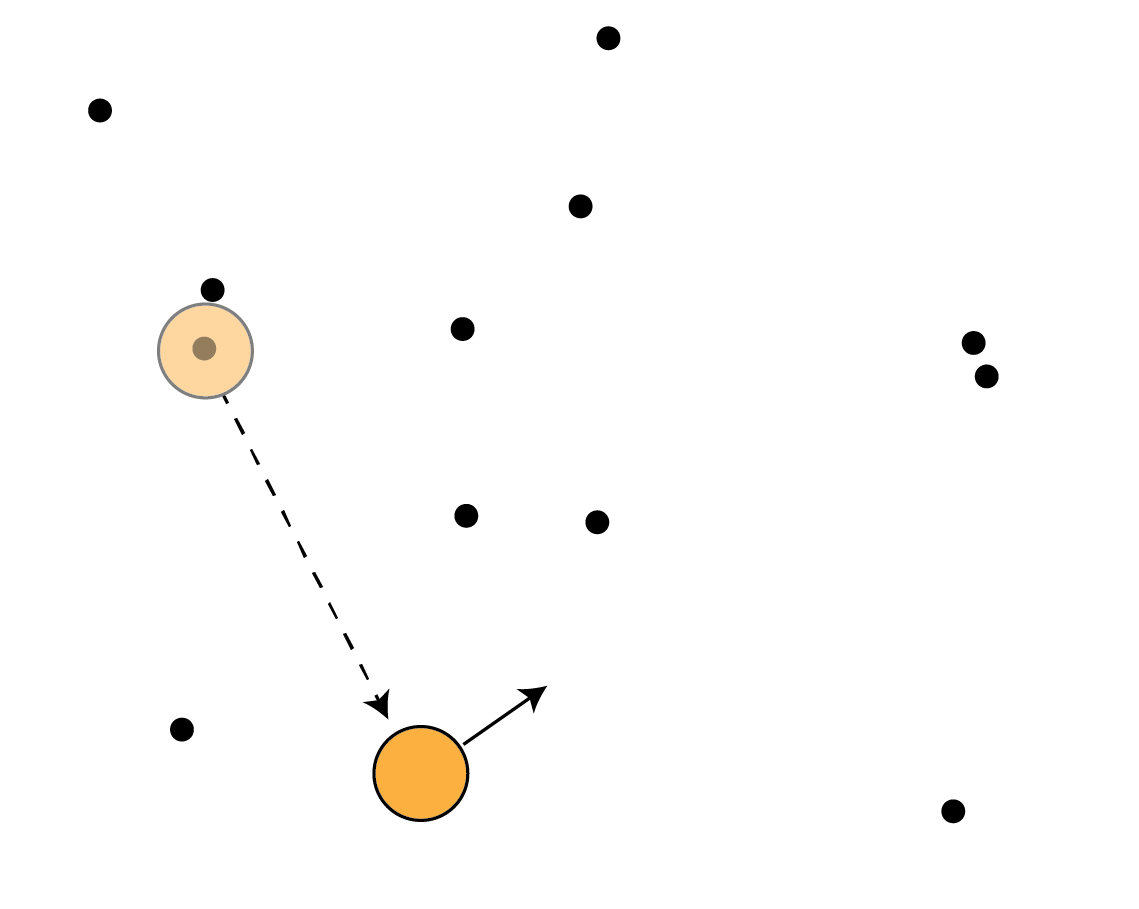
Problems:
- Can cells tune speed and persistence independently?
Can cells tune speed and persistence independently?
Migrating cells: universal coupling between speed and persistence (UCSP)
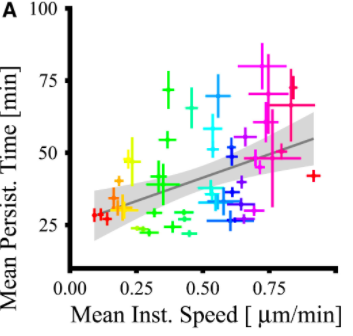
Maiuri et al, Cell 2015.
Can cells tune speed and persistence independently?
Migrating cells: universal coupling between speed and persistence (UCSP)

Maiuri et al, Cell 2015.
Maiuri et al, Cell 2015.
How T cells search

Problems:
- Can cells tune speed and persistence independently?
- Is behavior cell-intrinsic, or shaped by environment?
How do intrinsic (UCSP) and extrinsic (tissue) factors
constrain T cell migration patterns?
Approach: Modeling T cell migration in silico
This model must:
| 1 | describe migration | $\rightarrow$ T cells should actively move |
| 2 | reproduce UCSP | $\rightarrow$ speed/persistence as output, not input |
| 3 | be spatial | $\rightarrow$ T cells should have a shape |
| 4 | be multicellular | $\rightarrow$ allow cells to interact with surrounding tissue |
The Cellular Potts Model (CPM)
Pixels belong to cells, which
move by copying pixels:
Copy success chance (Pcopy) is higher when it helps the cell:
| Stay together: | Maintain its size: | Maintain its membrane: |
| $\searrow$ | $\downarrow$ | $\swarrow$ |
Approach: Modeling T cell migration in silico
This model must:
| 1 | describe migration | $\rightarrow$ T cells should actively move |
| 2 | reproduce UCSP | $\rightarrow$ speed/persistence as output, not input |
| 3 | be spatial | $\rightarrow$ T cells should have a shape |
| 4 | be multicellular | $\rightarrow$ allow cells to interact with surrounding tissue |
Migration: Act model
Cells move if we add positive feedback on protrusive activity ($\approx$ actin polymerization)1:
| Parameters: | ||
| λact | $\approx$ | protrusive force |
| maxact | $\approx$ | polymerized actin lifetime |
1Niculescu et al. PLoS Computational Biology, 2015.
Approach: Modeling T cell migration in silico
This model must:
| 1 | describe migration | $\rightarrow$ T cells should actively move |
| 2 | reproduce UCSP | $\rightarrow$ speed/persistence as output, not input |
| 3 | be spatial | $\rightarrow$ T cells should have a shape |
| 4 | be multicellular | $\rightarrow$ allow cells to interact with surrounding tissue |
Set-up: microchannels ("1D")
In silico microchannel:
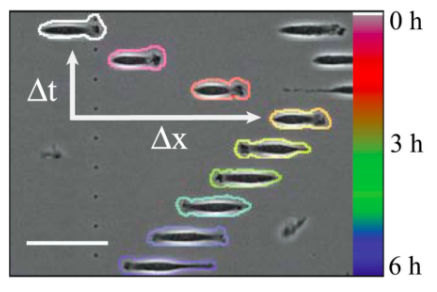
Maiuri et al, Cell 2015.
Set-up: 2D and 3D
Speed-persistence coupling emerges in the Act model
All tested λact / maxact combinations:
$\rightarrow$ Speed-persistence coupling exists in all 3 settings
Speed-persistence coupling emerges in the Act model
Similar cells grouped (2D and 3D):
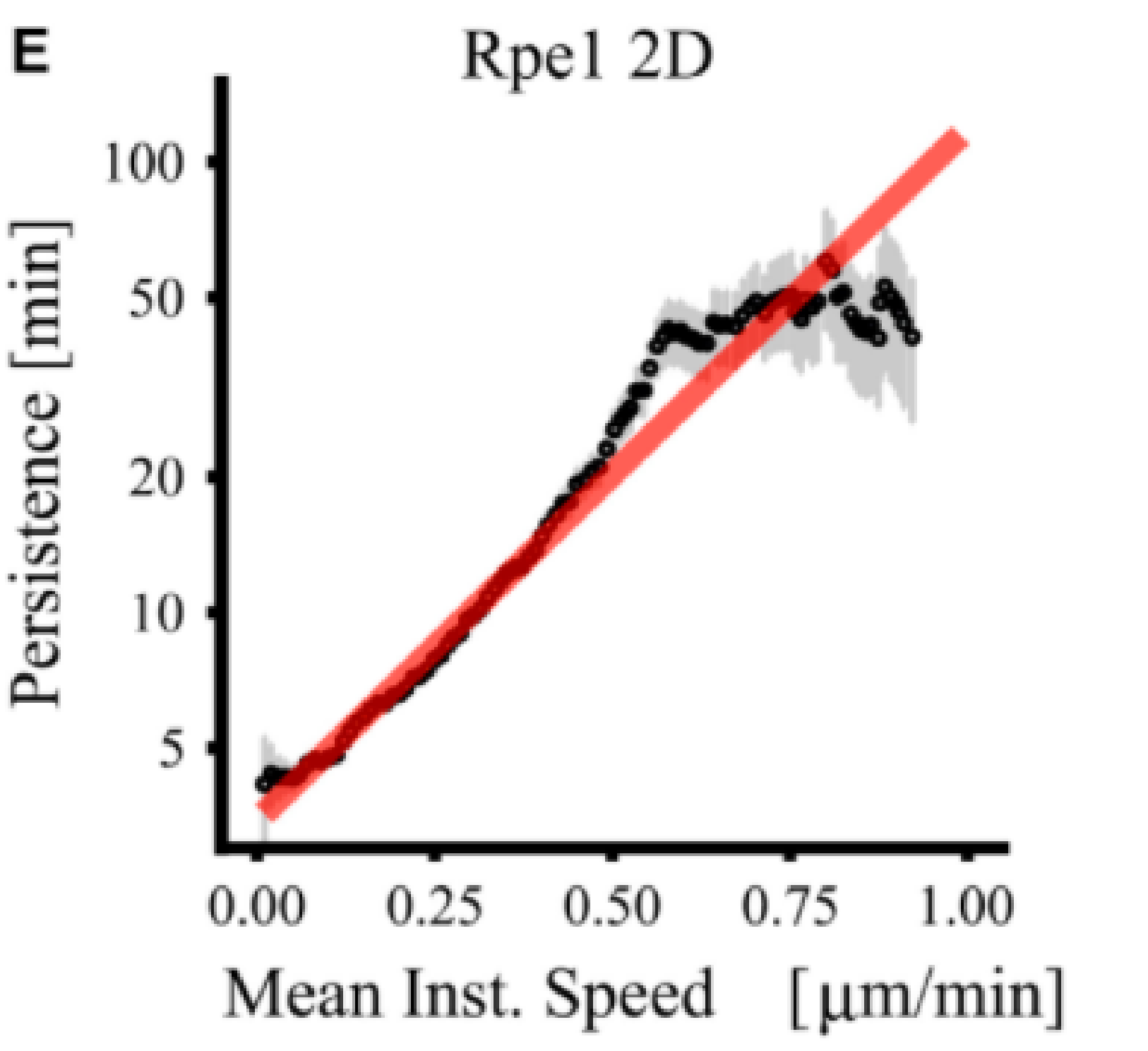 Maiuri et al, Cell 2015. Maiuri et al, Cell 2015. |
$\rightarrow$ Exponential coupling is strong in cells with same maxact
Speed-persistence coupling spans different migration modes
Similar cells grouped (2D and 3D):
$\rightarrow$ Coupling holds throughout different shapes & behaviors
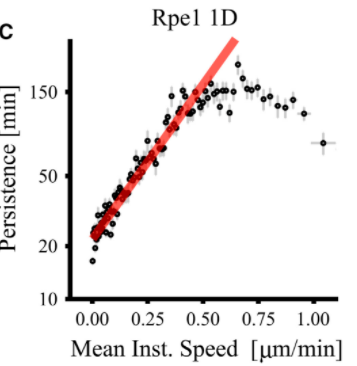
Persistence saturates at high λact
Model:
Experimental data:
$\rightarrow$ Model reproduces saturation observed in vitro
Both speed and persistence have an upper bound
At some point, the cell cannot go faster or straighter:
Shape changes drive speed/persistence saturation
Microchannels restrict shape changes:
... And indeed do not show saturation:
Back to T cells: the epidermis
The epidermis is packed tightly with keratinocytes
...Yet T cells remaining after an infection are highly motile:
 |
|
| Ariotti et al, PNAS 2012. |
$\rightarrow$ Can we still see the UCSP for T cells in such a dense environment?
Act T cells migrate between keratinocytes in the epidermis
maxact = 30:
"stop-and-go"
maxact = 100:
protrusions split
T cells cannot move straight in the epidermis
In a tightly packed skin:
$\rightarrow$ Stringent environmental constraints can overrule the UCSP
What about more deformable tissue?
Higher persistence is possible, but there still is a limit:
Summary I: constraints on T cell motility
Cells cannot freely "choose" their speed and persistence:
| - | Speed and persistence are coupled (cell-intrinsic) |
| - | Environmental constraints further restrict motility patterns, and can overrule the UCSP (cell-extrinsic) |
$\rightarrow$ "Optimizing" speed and persistence is not so straightforward!


"Evolving" super searchers: what search behavior can we get?
Use a genetic algorithm to let cells maximize the area they explore. Each generation, cells undergo three phases:
Both maxact and λact evolve towards high values
Both maxact and λact evolve towards high values
Both maxact and λact evolve towards high values
Cells evolve towards high speed and persistence
| Before: | |
| After: |
Skin-constrained cells evolve to similar parameters...
Skin-constrained cells evolve to similar parameters...
... But different behavior!
| Free: | |
| Skin: |
Summary II: evolution of "optimal" search patterns?
Cells can "optimize" their search to some extent, but:
| - | Even if we make evolution easy, the cell is still subject to constraints and trade-offs |
| - | What is "optimal" depends both on the cell and the environment |
| - | Cells with very similar parameters can look very different depending on the environment they're in |
$\rightarrow$ Do cells choose their speed and persistence, or does their environment choose it for them?


Part II
When models meet data:
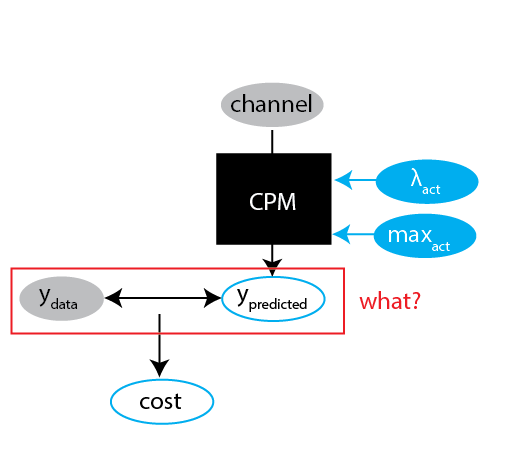
Can we fit the CPM to "real" migration movies?


Why fit a CPM model?
We can get different migratory patterns by varying λact and
maxact (and some other parameters).
- Different parameters = different behavior
- When mimicking "real" cells and making predictions, how do we know which parameter values to pick?
Why fit a CPM model?
Up till now: manual tuning until CPM cells "look like" real cells.
This is a problem because...
- ... it's boring
- ... it's not very transparent/reproducible.
Can we do better?

Model fitting: an easy example
The problem:
- Points $(x,y)$
- Input: $x$
- Output: $y$
Based on these data, can we come up with a model that can predict $y$ for new, unseen $x$?
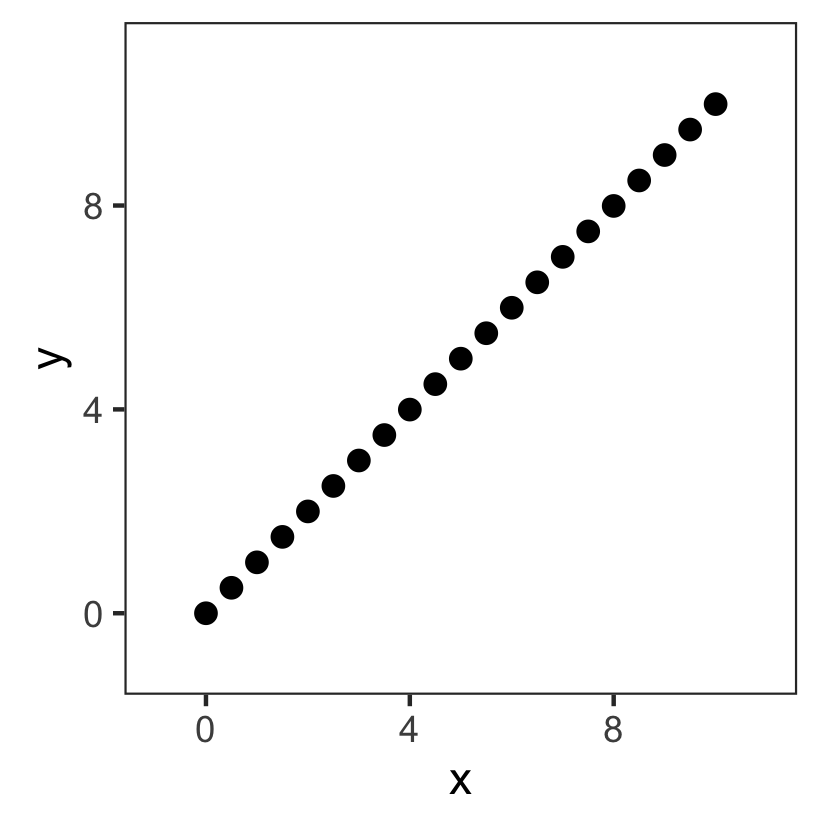
Model fitting: an easy example
Choosing a model:
- Linear model with $y = ax + b$
- Parameters are $a$ and $b$
The problem now becomes: what are $a$ and $b$? Finding these values is called model fitting.
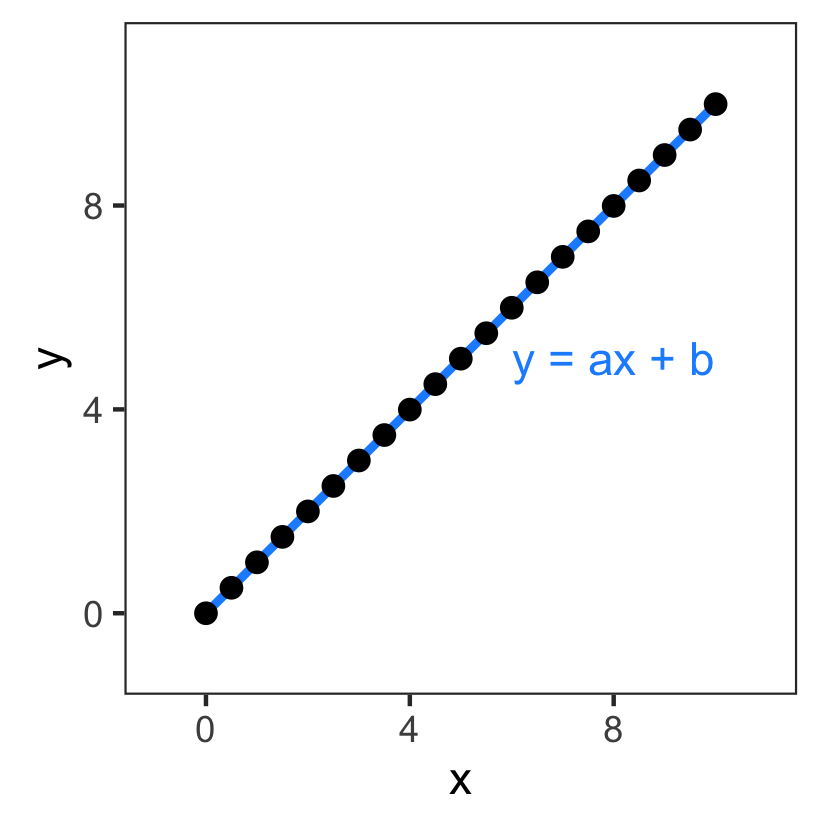
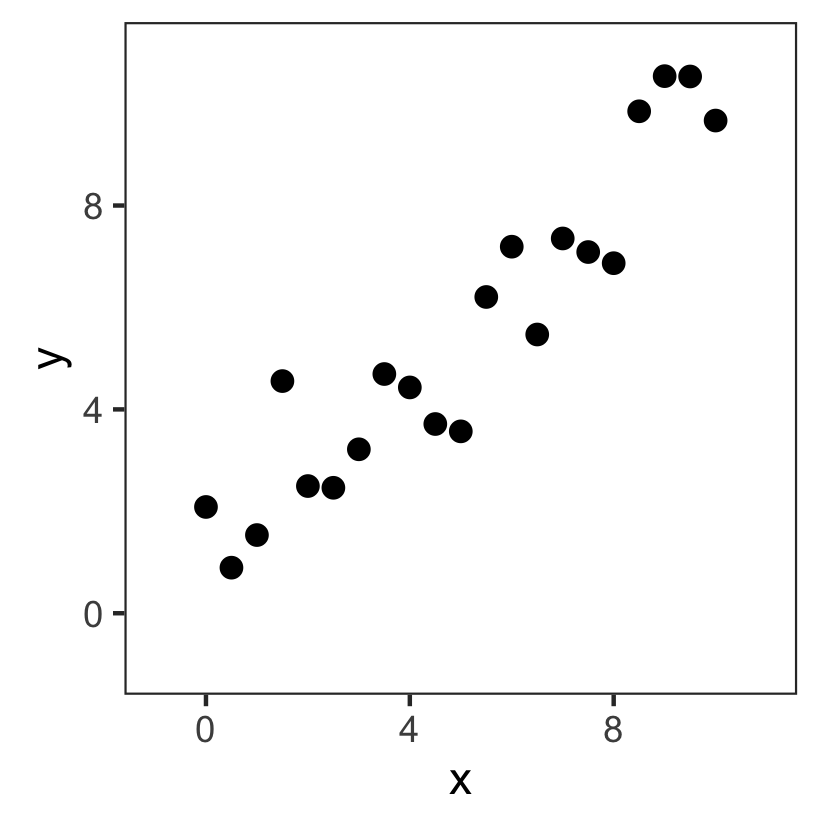
Even this easy example is not so easy...
If there is noise, how do we choose which $a$ and $b$ are best?
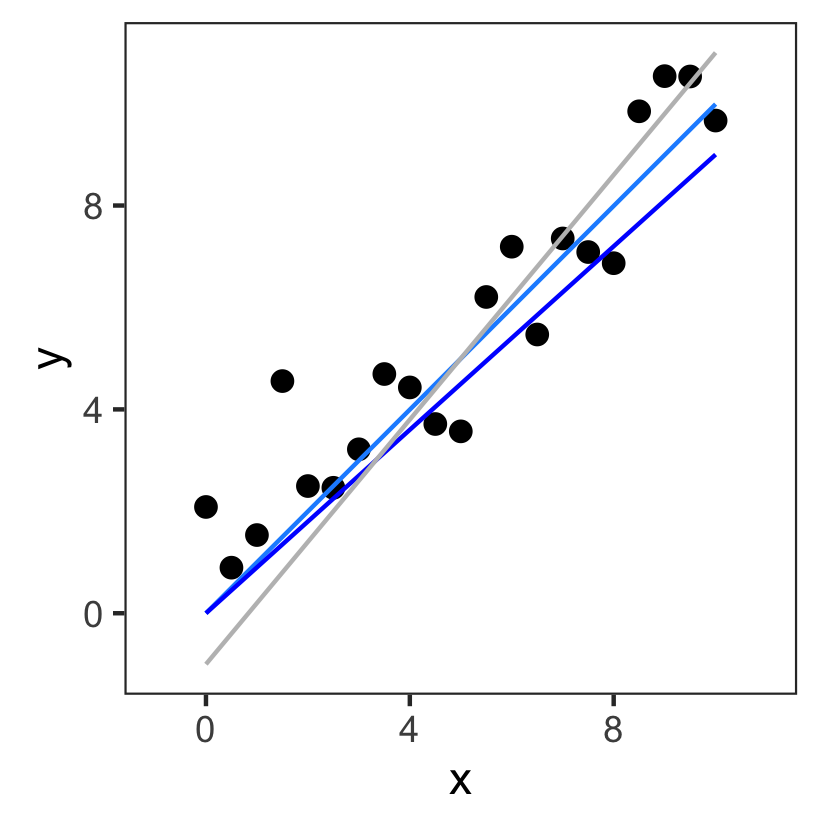
Ordinary Least Squares Regression
The "best" model is the one with the smallest sum of squares:
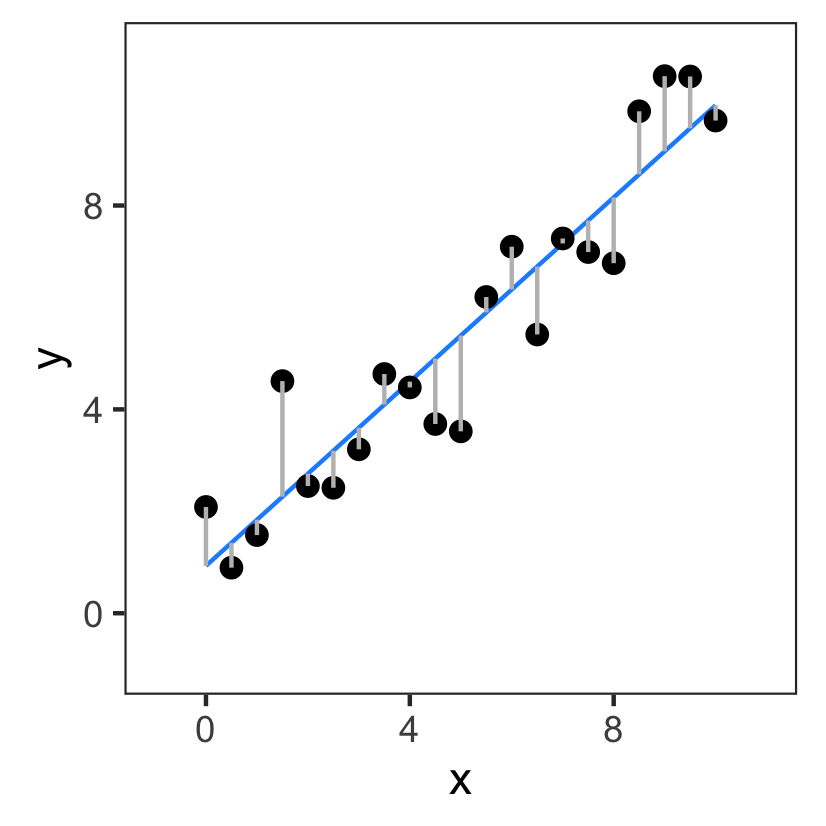
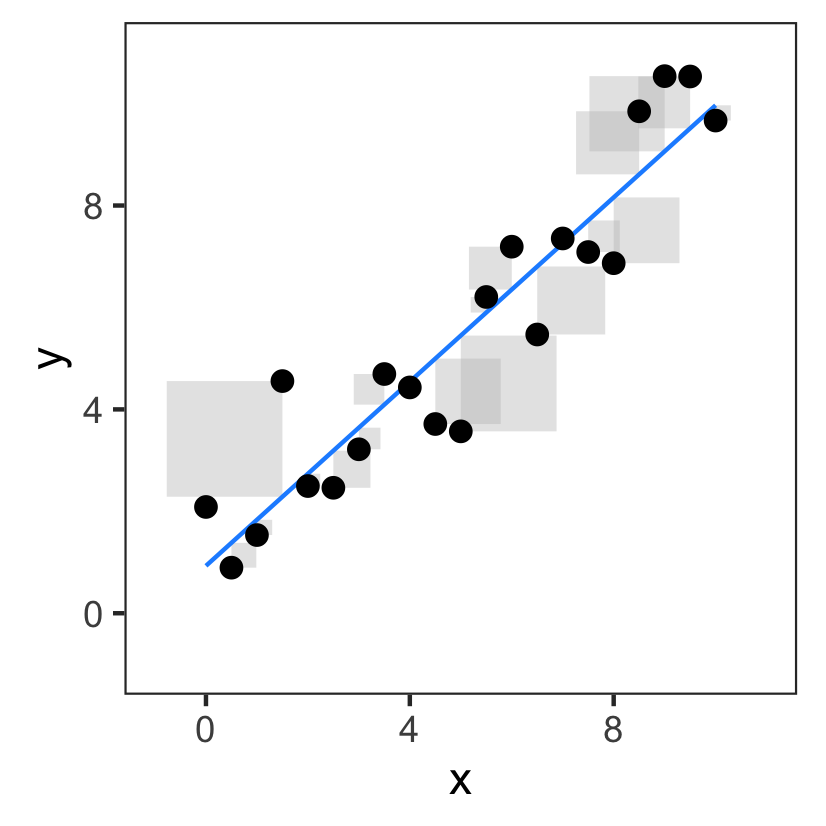
Ordinary Least Squares Regression
A better fit has a smaller sum of squares:
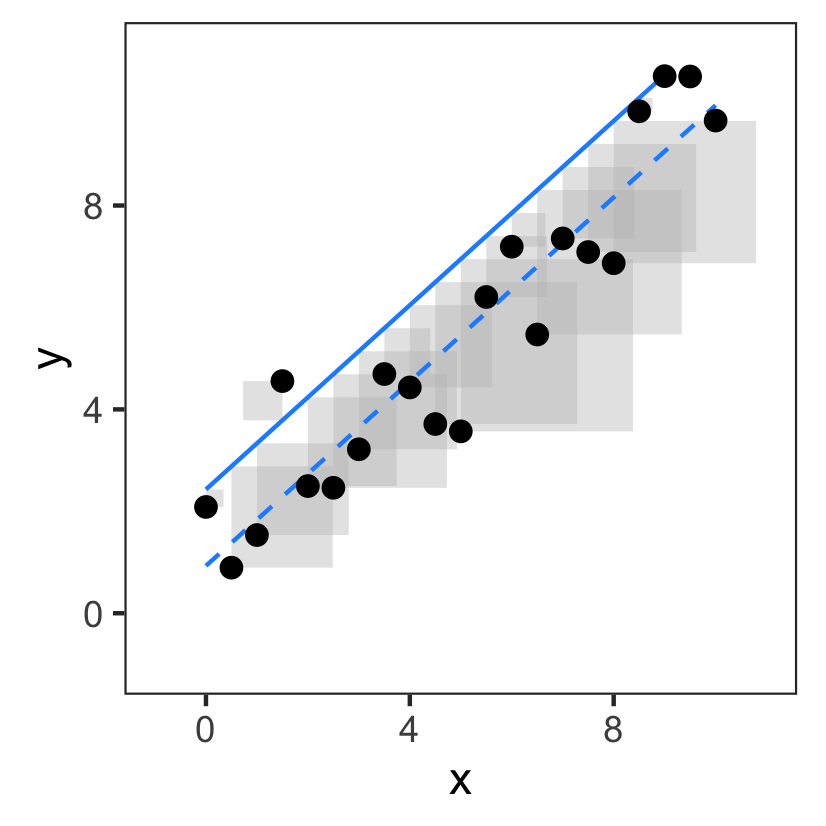

So the sum of squares is a cost we try to minimize.
Fitting algorithm
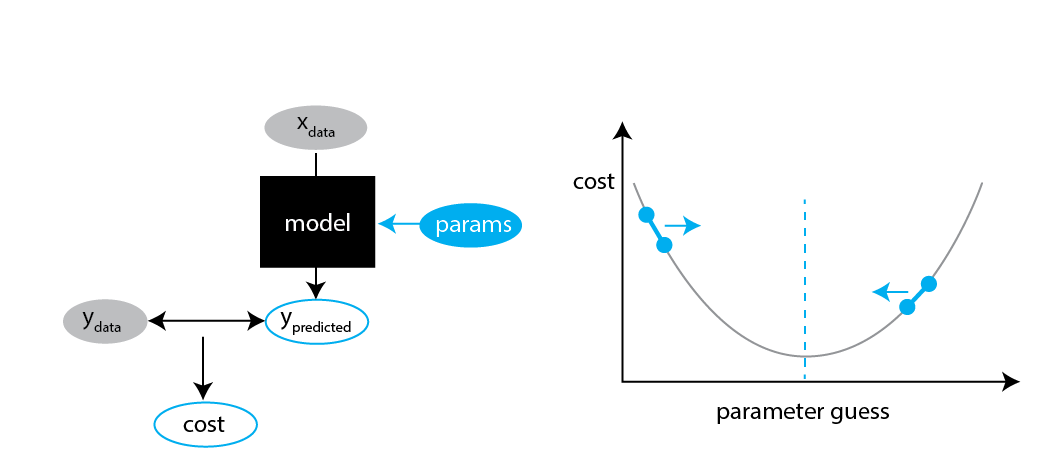
We can now "fit" our optimal parameters: guess parameters, evaluate cost, and go in the direction where the cost decreases.
Fitting multiple parameters
Remember, we had two parameters $a$ and $b$:
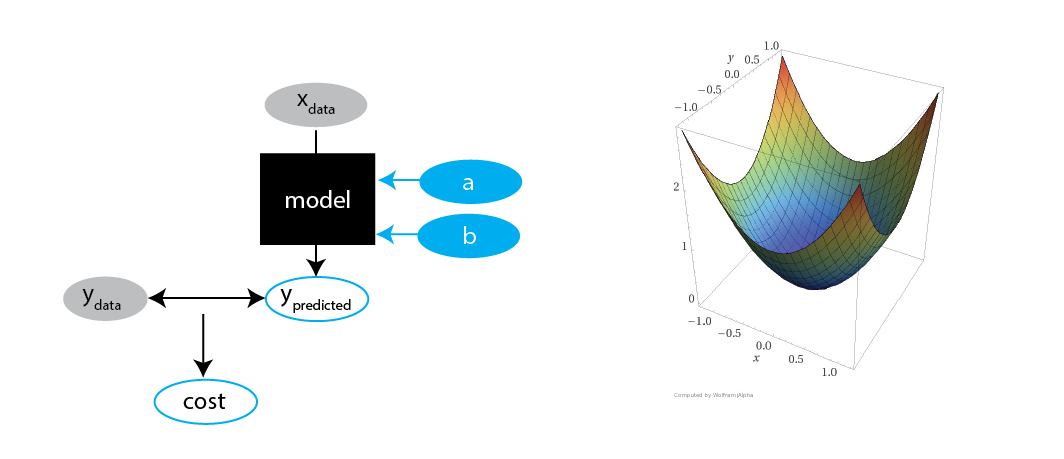
Challenge 1: definition of cost
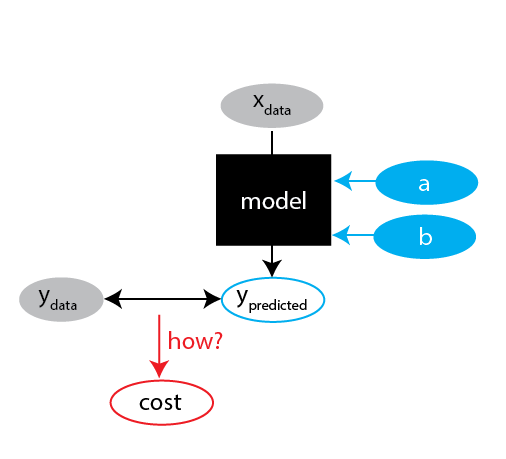

The fitted values we get depends on how we define the cost.
Challenge 1: definition of cost


The fitted values we get depends on how we define the cost.
Challenge 1: definition of cost

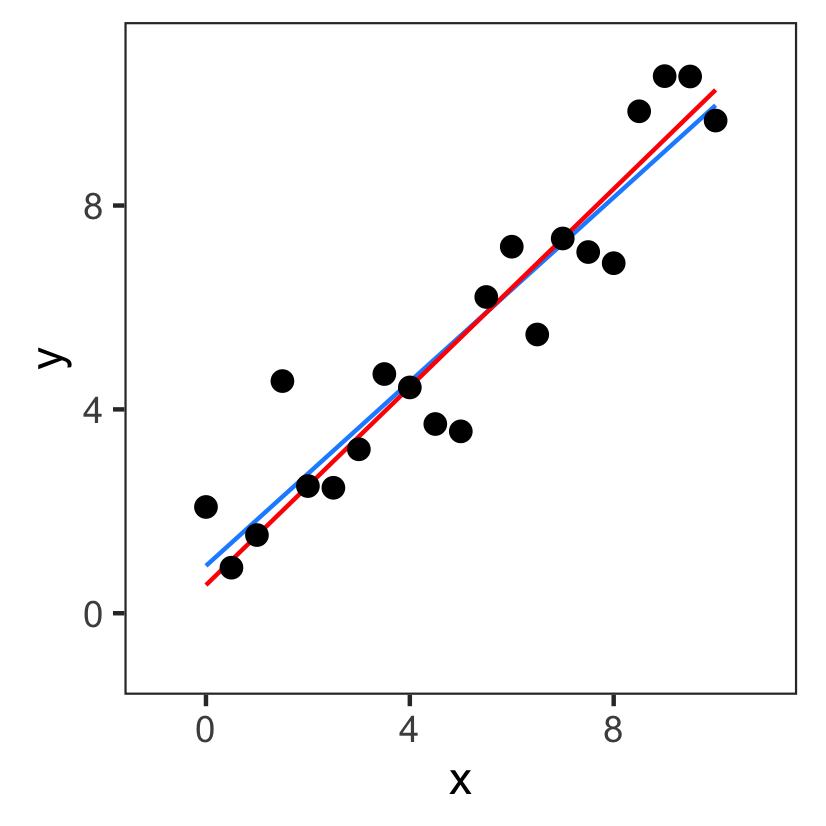
The fitted values we get depends on how we define the cost.
Challenge 1: definition of cost

The fitted values we get depends on how we define the cost.
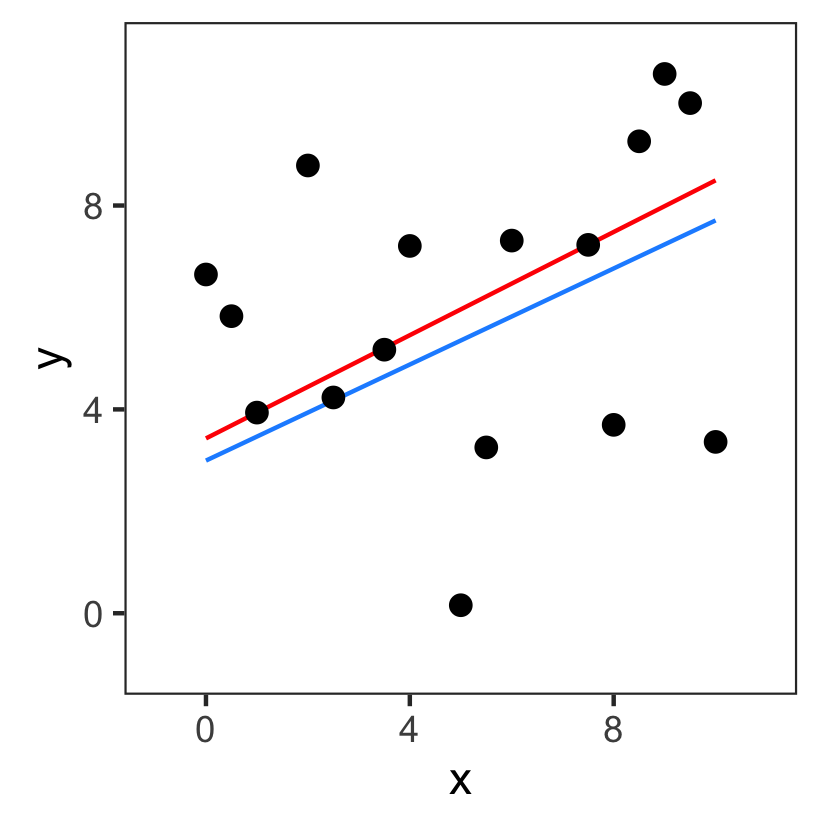
Challenge 2: what are we fitting, exactly?
- Speed?
- Persistence?
- Cell shape?
- Cell breaking?
- Relative importance?
Challenge 2: what are we fitting, exactly?

Fit the (mean) squared displacement:
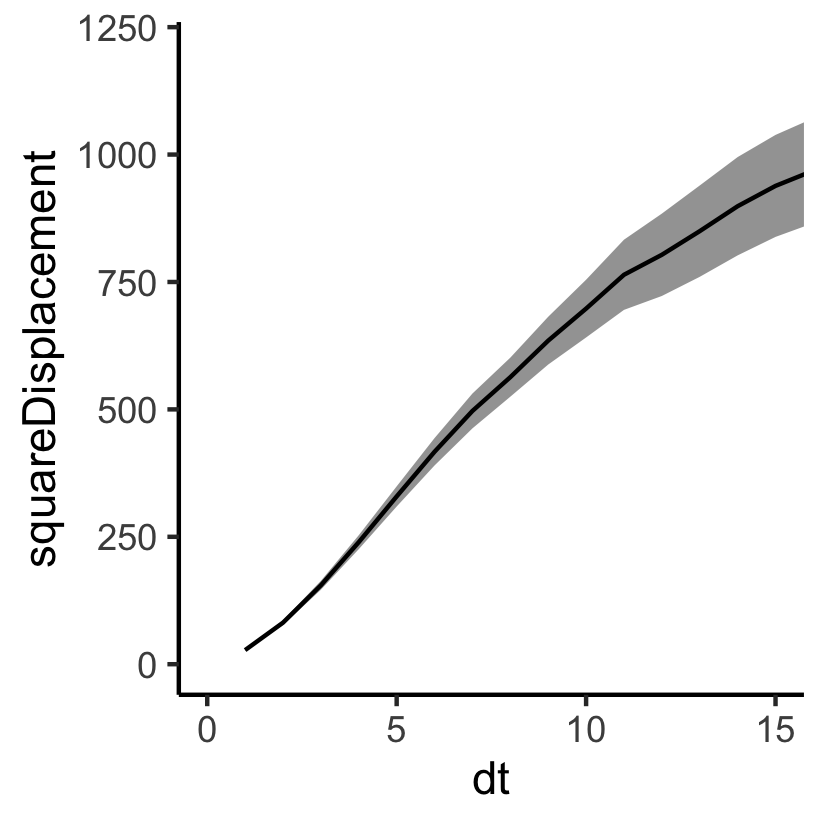
Challenge 2: what are we fitting, exactly?

Fit the log squared displacement:
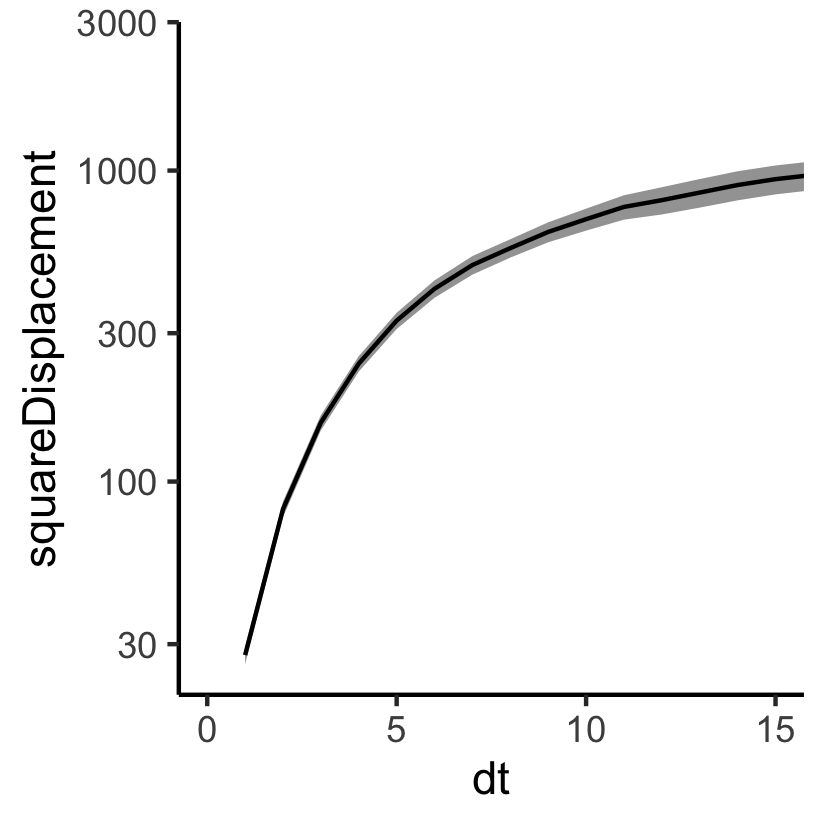
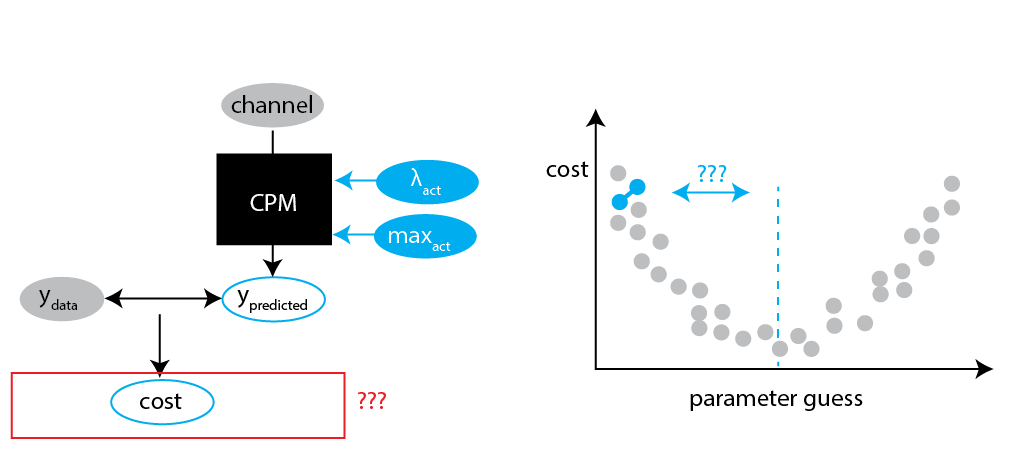
Challenge 3: do we know our cost?
If we run the CPM multiple times with the same parameters, we get different output...
Fitting the CPM on MSD
Fitting the CPM on MSD
Before and after fitting:
Summary
Fitting CPMs is difficult:
- Result depends on many choices
- We have to deal with noise
- We have to choose what we're fitting
Fitting CPMs is possible:
- We can fit an MSD plot reasonably well (and fast)!
- Future directions: improve cell shape, fit more parameters, try different "outputs".
Summary
Fitting CPMs is difficult:
- Result depends on many choices
- We have to deal with noise
- We have to choose what we're fitting
Fitting CPMs is possible:
- We can fit an MSD plot reasonably well (and fast)!
- Future directions: improve cell shape, fit more parameters, try different "outputs".

Acknowledgments
Tumor Immunology, Nijmegen
- Johannes Textor
- Jolanda de Vries
- Carl Figdor
- Human DLM
Department of Physiology, McGill, Canada
- Judith Mandl
- Jérémy Postat
Funding
- Radboudumc PhD grant
Theoretical Biology, Utrecht
- Rob de Boer
- Martijn Kolijn
- Ioana Niculescu
Chemical & Biological Physics, Weizmann Institute, Israel
- Nir Gov


Questions?

