BIRS, May 5th, 2022
Mechanistic modelling of cell migration
in the immune system
"Transparent modelling" beyond ML
Inge Wortel
Computational Immunology group,
Radboud University Nijmegen, NL
inge.wortel@ru.nl![]()
@inge_wortel![]()
The biological problem.
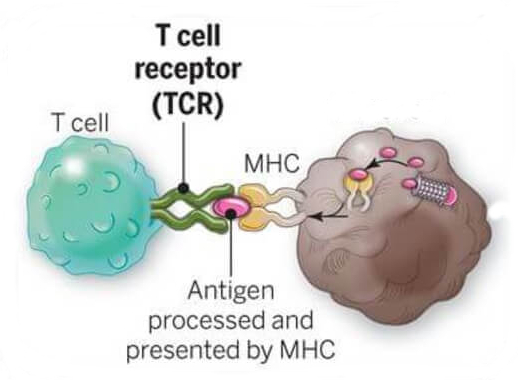
T cells as highly specialized anomaly detectors.
 Adapted from National Cancer Institute (NIH)
Adapted from National Cancer Institute (NIH)

T cells:
- Detect and clean up infected/cancerous cells
- Using their T-cell receptor (TCR) to screen for short peptides displayed on a molecule called MHC
- Compromised (infected/cancerous) cells display different peptides than healthy cells do
$\rightarrow$ "anomaly detection" - Specific because each T cell's receptor recognises specific peptides
T cells search for anomalies in lymph nodes.
Only one in a million T cells can detect any given new pathogenic signal (peptide).
T cells must search for these rare targets that can activate them.
They do this in central "meeting hubs" called lymph nodes.
A. Peixoto, Harvard Medical School
Hidden figures.
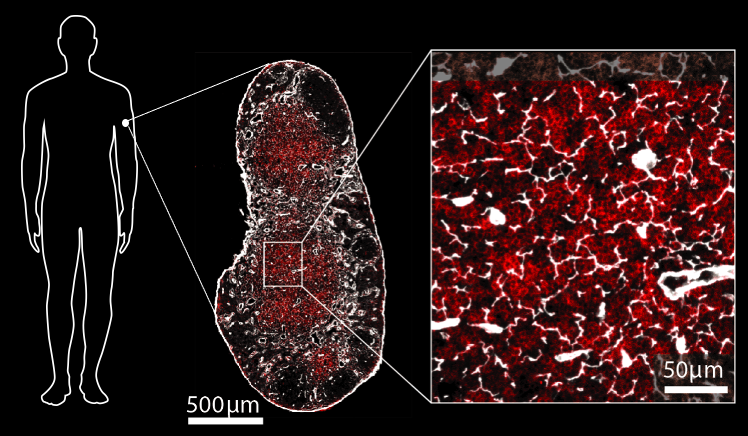
Image: Connie Shen & Judith Mandl.
The question: why no traffic jams?

|
? |
How do T cells respond to complex and crowded environments, and does their smooth traffic flow ever break down?
mechanistic
modelling.

"What I cannot create, I do not understand."
— Richard Feynman
"Creating" real and simulated T-cell crowds.
Put T cells in controlled environments, inspired by the physics field of crowd dynamics.
- In silico: computational model
- In vitro: controlled environment in the lab
Can we "build a crowd" — i.e., can our model predict what real T cells will do in vitro?
Data: time-lapse imaging of moving cells. Predictions: what will the crowd do?
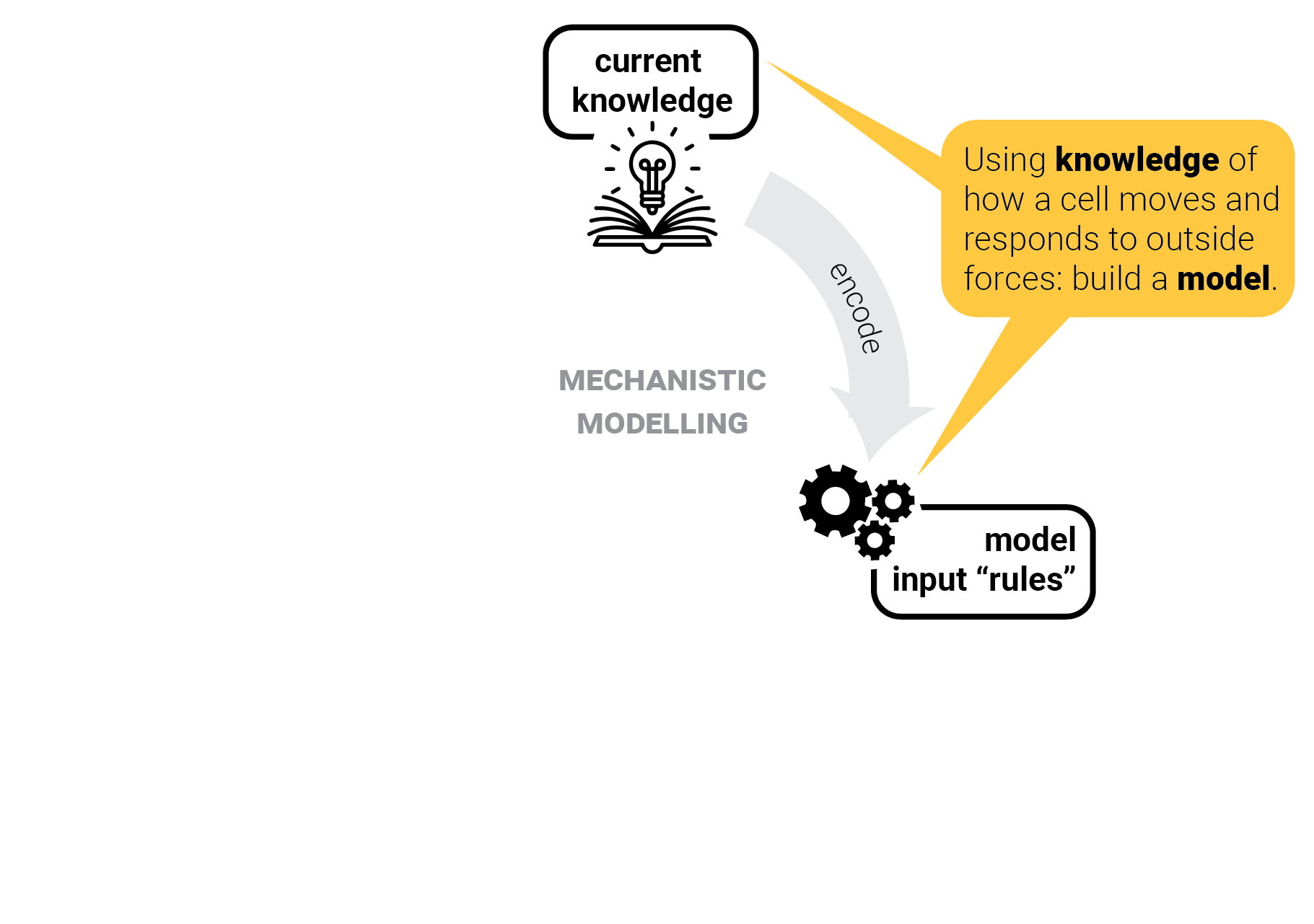
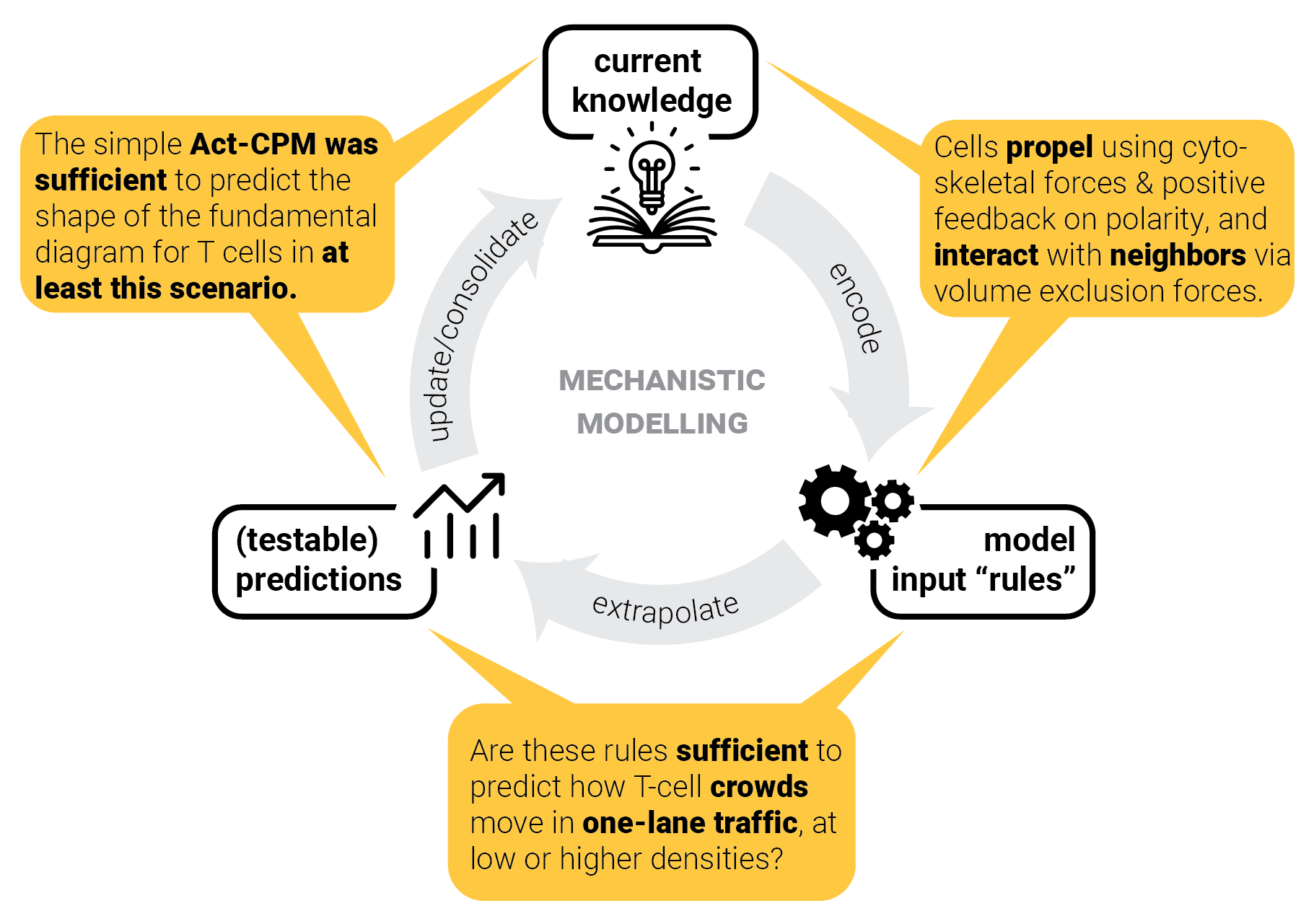
The circle of life mechanistic modelling.

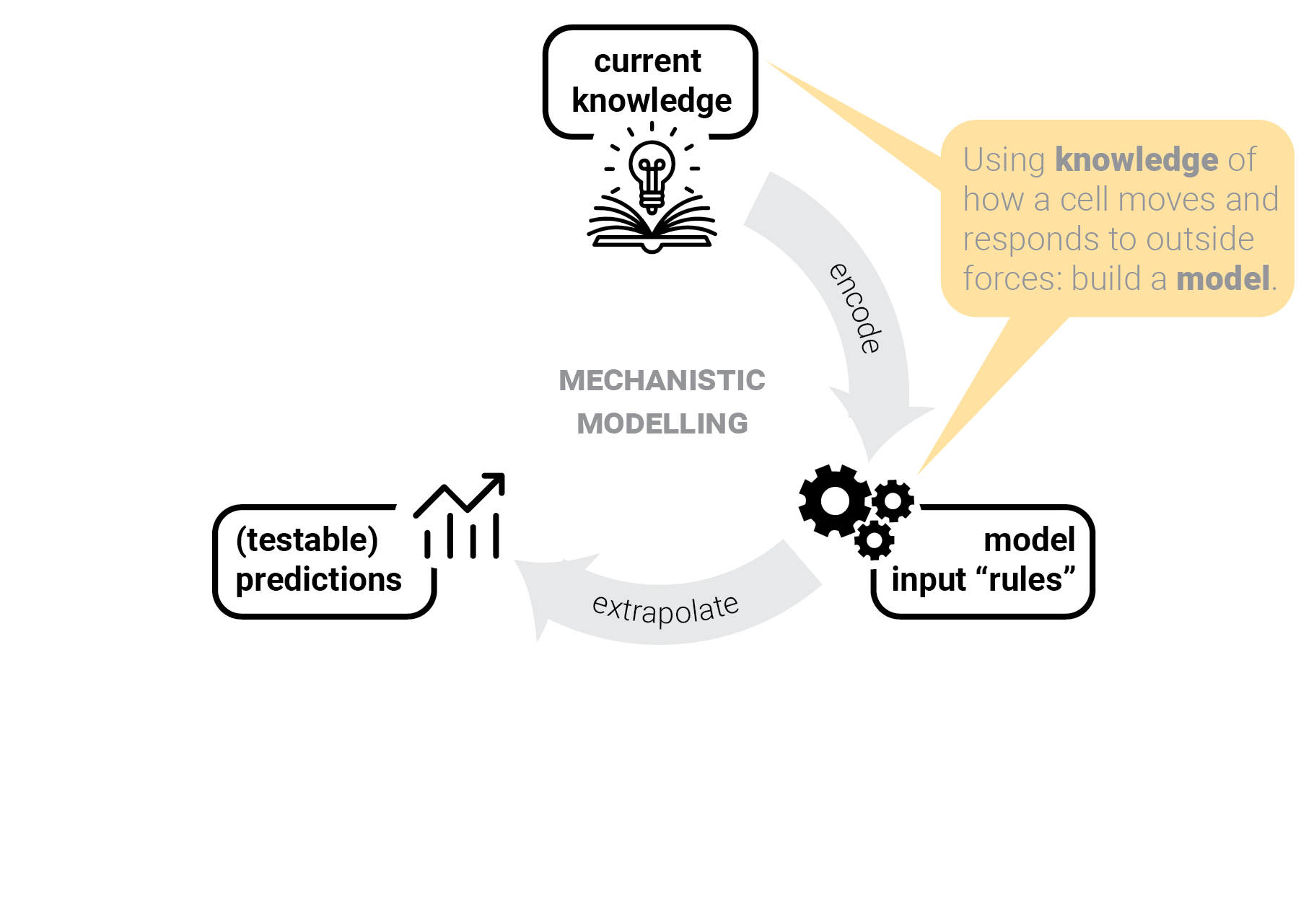
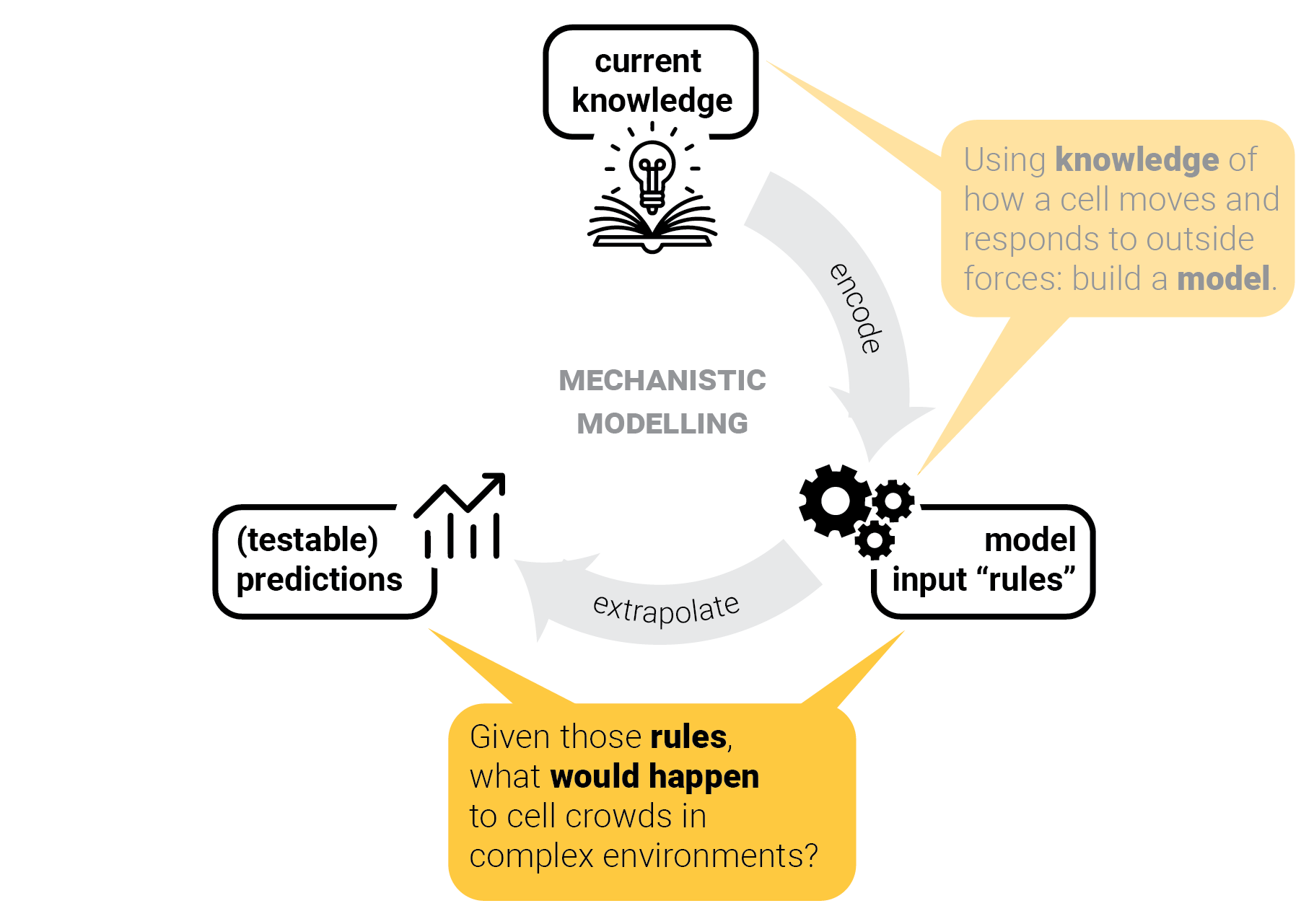
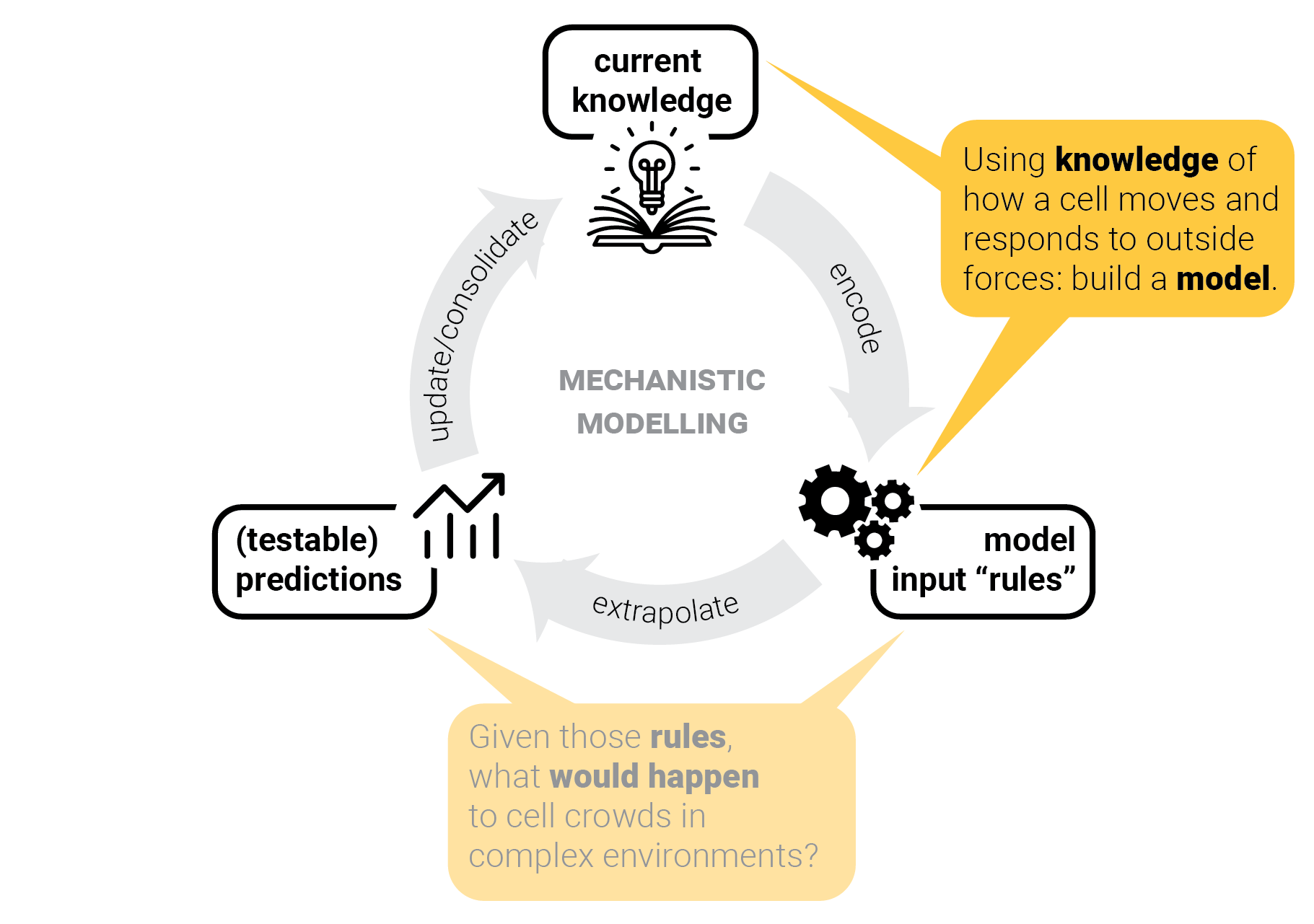
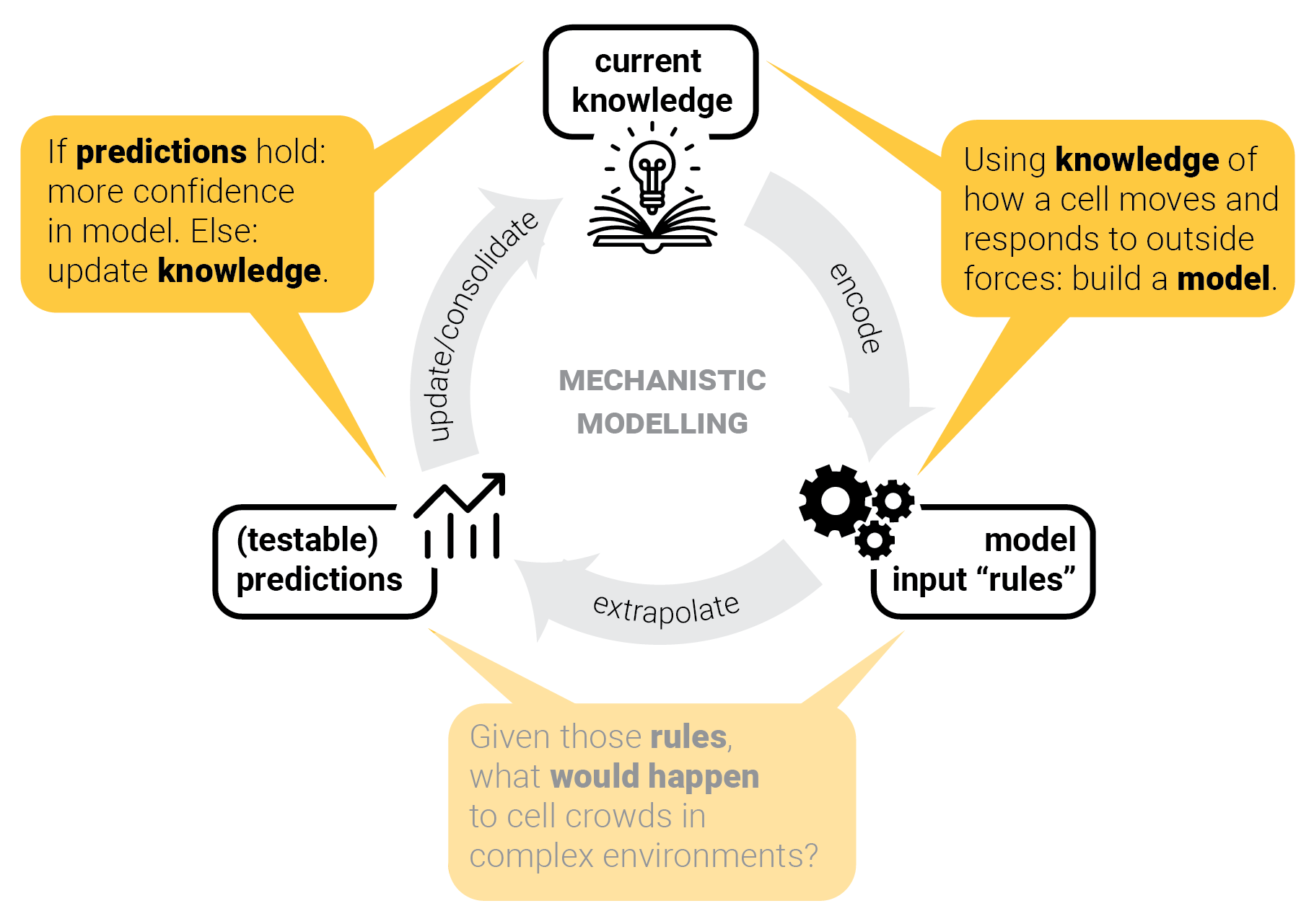
The role of predictions.
(Interpretable) AI
| Good predictions/decisions | |
| Knowledge/models | |
| Black-box models: OK (if we could be sure they were trustworthy & fair) |
|
| Interpretability is a side-goal to foster trust, fairness, accuracy. |
Mechanistic modelling
| Knowledge (or models of it) | |
| Predictions | |
| Black-box models: no knowledge gain (since we don't know how they work) | |
| Interpretability is critical to extract knowledge from mechanistic models. |
T-cells in one lane traffic.
Step 1: gather input knowledge.
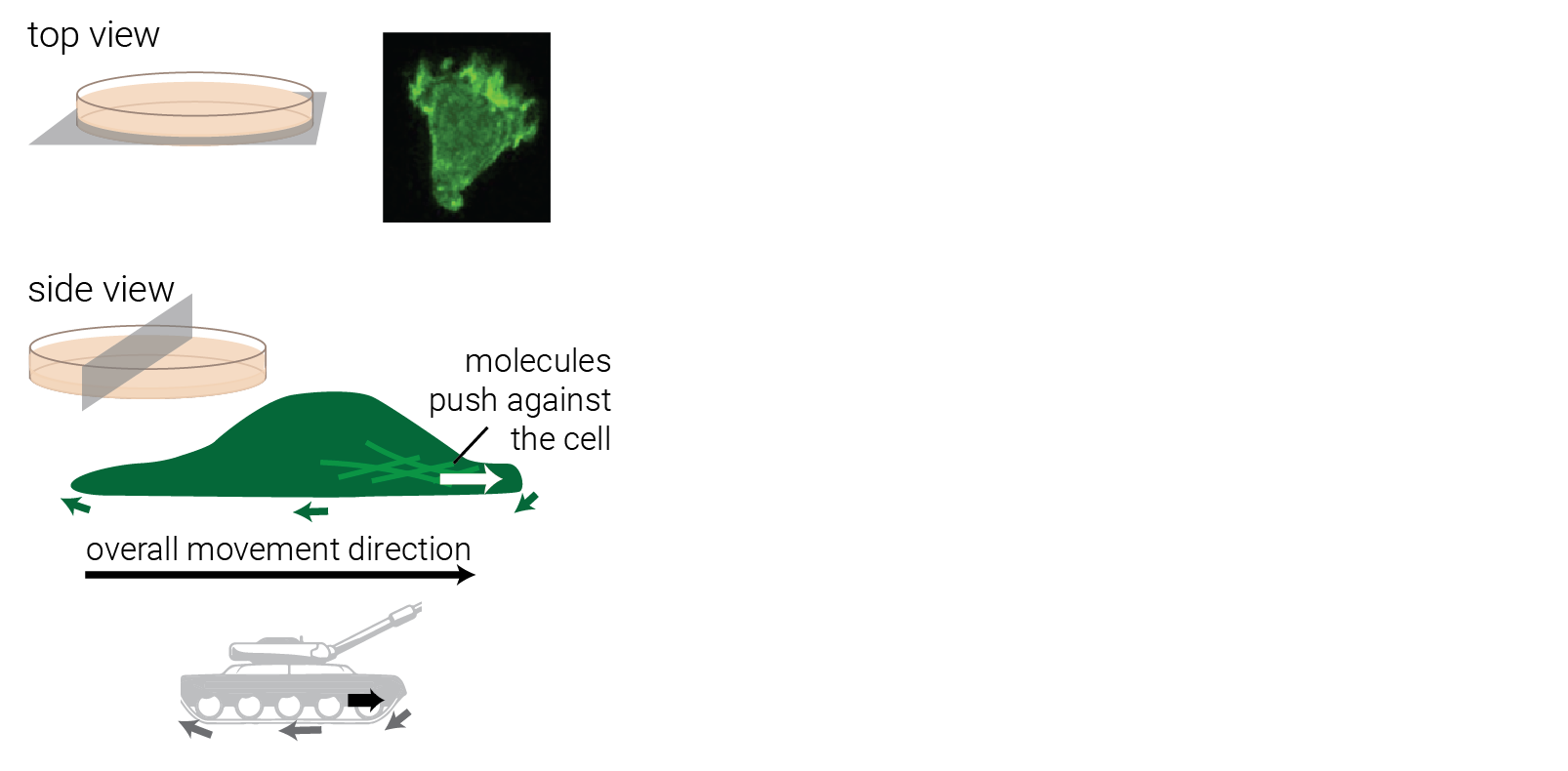
Step 1: gather input knowledge.
Step 2: encode into a model.
Option 1: Detailed model
Explicitly encode every molecule and resulting force.
+ highly interpretable!
+ emergent behavior.
— too expensive to model crowds.
Step 2: encode into a model.
Option 2: Phenomenological — Cellular Potts Model (CPM)1
Pixels belong to cells, which
move by copying pixels:
Copy success chance (Pcopy) is higher when it helps the cell:
Powered by Artistoo.net
$P_\text{copy} = \begin{cases} e^{-\Delta H/T} & \Delta H \gt 0\\ 1 & \Delta H \leq 0 \end{cases}$
$\rightarrow$ Cells have shapes and interact naturally through volume exclusion (each pixel can only belong to one cell at a time). Crowd behavior still emerges.
1Graner and Glazier (1992). doi:10.1103/PhysRevLett.69.2013
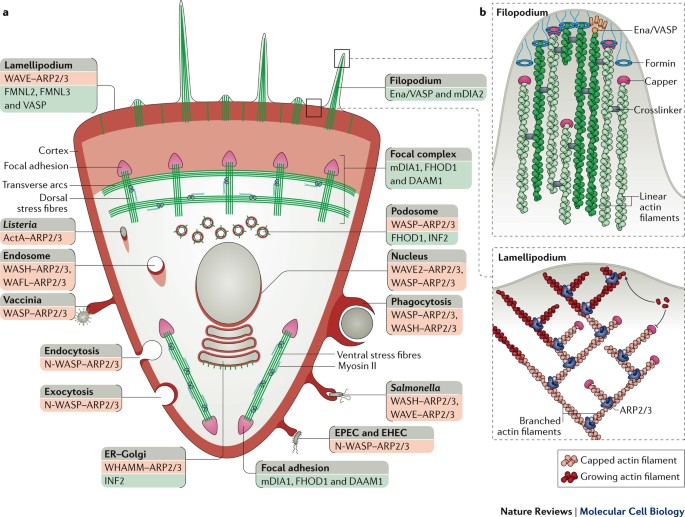
Step 2: encode into a model.
Powered by Artistoo.net
Cells move if we add positive feedback on protrusive activity ($\approx$ actin polymerization)1:
| Parameters: | ||
| λact | $\approx$ | protrusive force |
| maxact | $\approx$ | polymerized actin lifetime |
$\rightarrow$ realistic cell shape and motility 1,2.
1Niculescu et al. (2015). doi:10.1371/journal.pcbi.1004280
2Wortel et al. (2021). doi:10.1016/j.bpj.2021.04.036
Step 3: predict crowd behavior.
A cornerstone scenario in crowding physics: one-lane traffic.
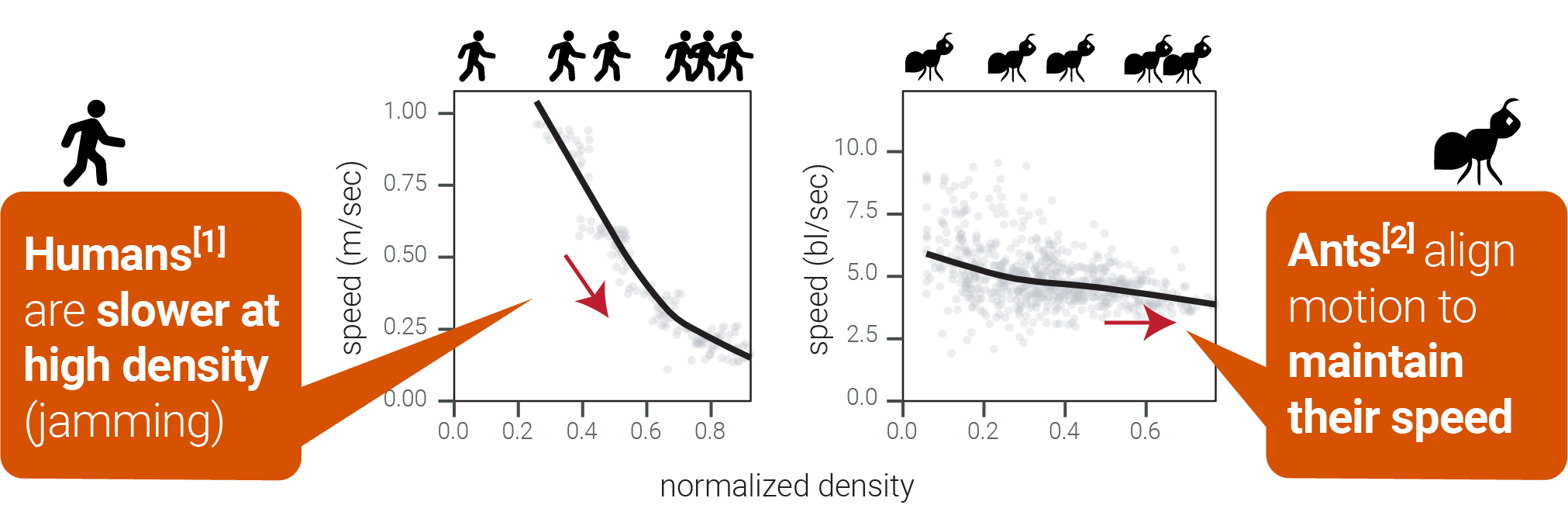
1John et al. (2009). doi:10.1103/PhysRevLett.102.108001
2Seyfried et al. (2005). doi:10.1088/1742-5468/2005/10/p10002
Step 3: predict crowd behavior.
What do T cells do? Put single (CPM) cells together in constrained channels and predict crowd behavior:
Qualitatively: cells rapidly align into "trains" to keep moving.
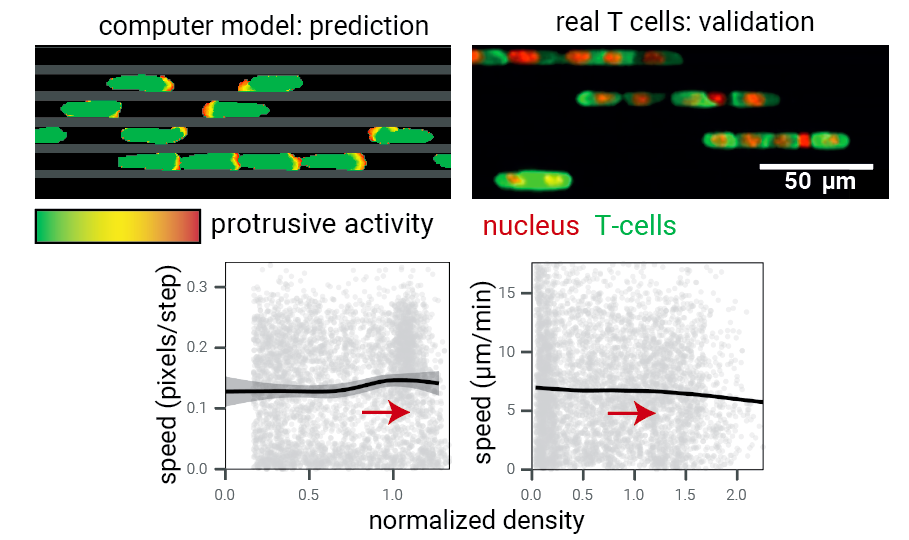
Step 4: test model predictions.
What about real T cells? Again: train formation!
Data: Jérémy Postat and Judith Mandl.
Step 4: test model predictions.
Quantitatively: the fundamental diagram in both cases is flat.
Step 5: consolidate model — and repeat.
Model consolidation != proof.
Can we predict crowd behavior in other scenarios as well?
Step 5: consolidate model — and repeat.
Pedestrian crowds can form jamming arches near an exit. This scenario is well-studied because of crowd disasters, such as at the Love Parade (Berlin, 2010).
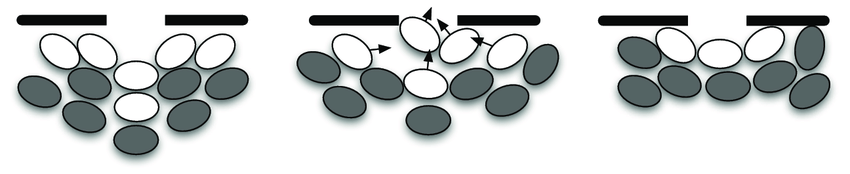
$\rightarrow$ What about T cells?
Step 5: consolidate model — and repeat.
Simulated T cells can indeed form jamming arches:
Work in progress, but see: Wortel (2021). https://repository.ubn.ru.nl/handle/2066/236680.
Work of Shabaz Sultan
Challenge:
Are CPMs interpretable?
CPMs are not fully interpretable.
Emergent behavior is nice, but...
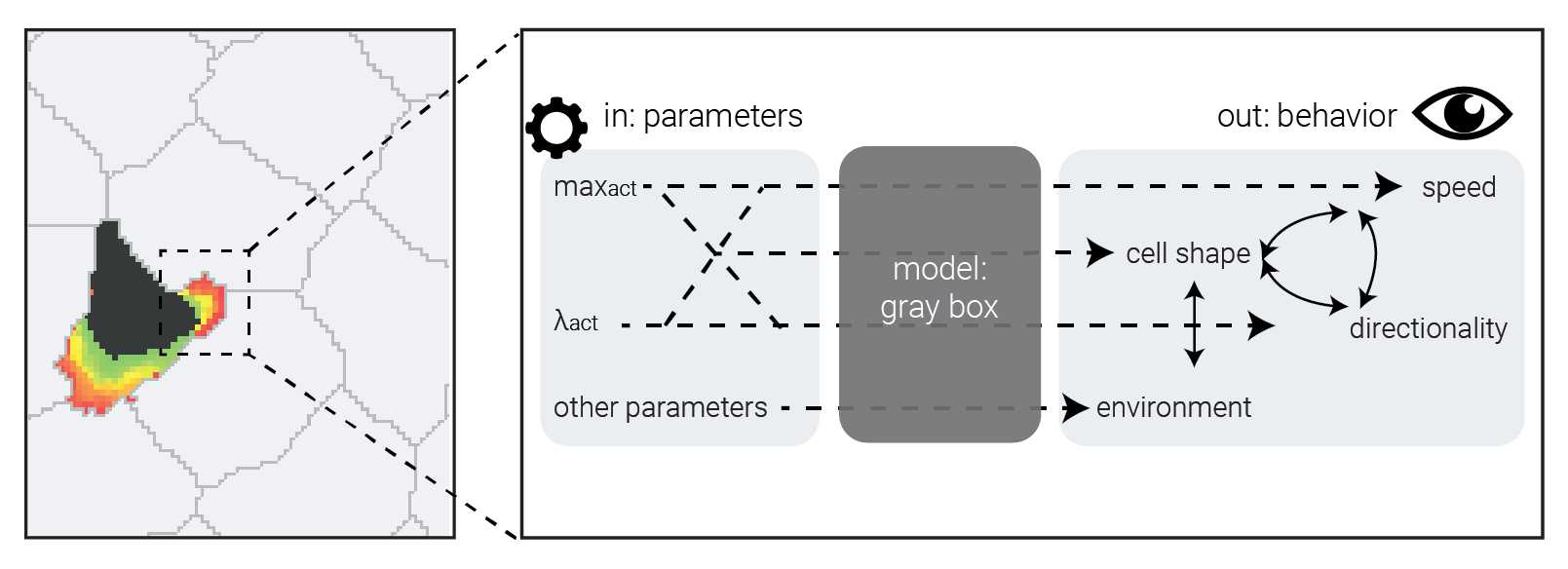
... we still don't know exactly how parameters lead to outputs.
"Explaining" CPMs — visualization
Visualizing and manipulating models interactively: artistoo.net

"Explaining" CPMs — tracking internal states
Tracking internal model states and outcomes over time:
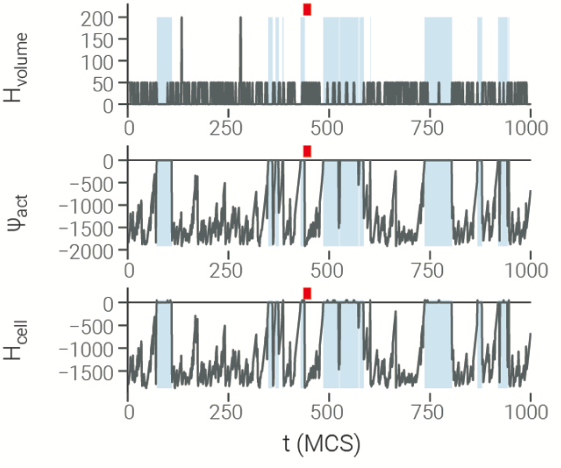
- competing energy terms
(i.e.: maintaining volume, adhesion, protrusions, ...) - protrusion activity
- cell breaking
- cell shape, speed, turning, ...

"Explaining" CPMs — parameter screening
For example: how does cell motion in a microchannel depend on channel size & cell flexibility (perimeter)?
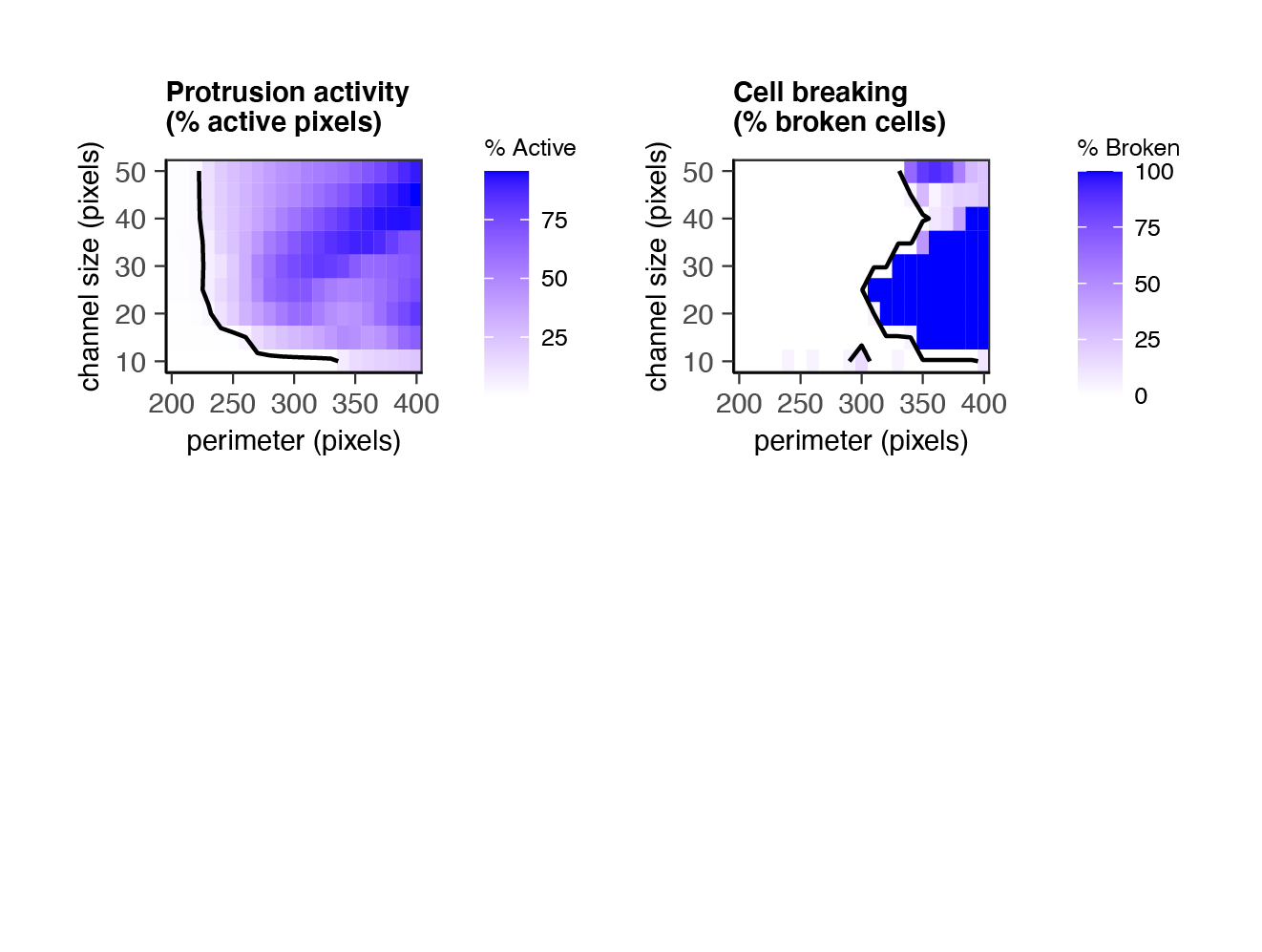
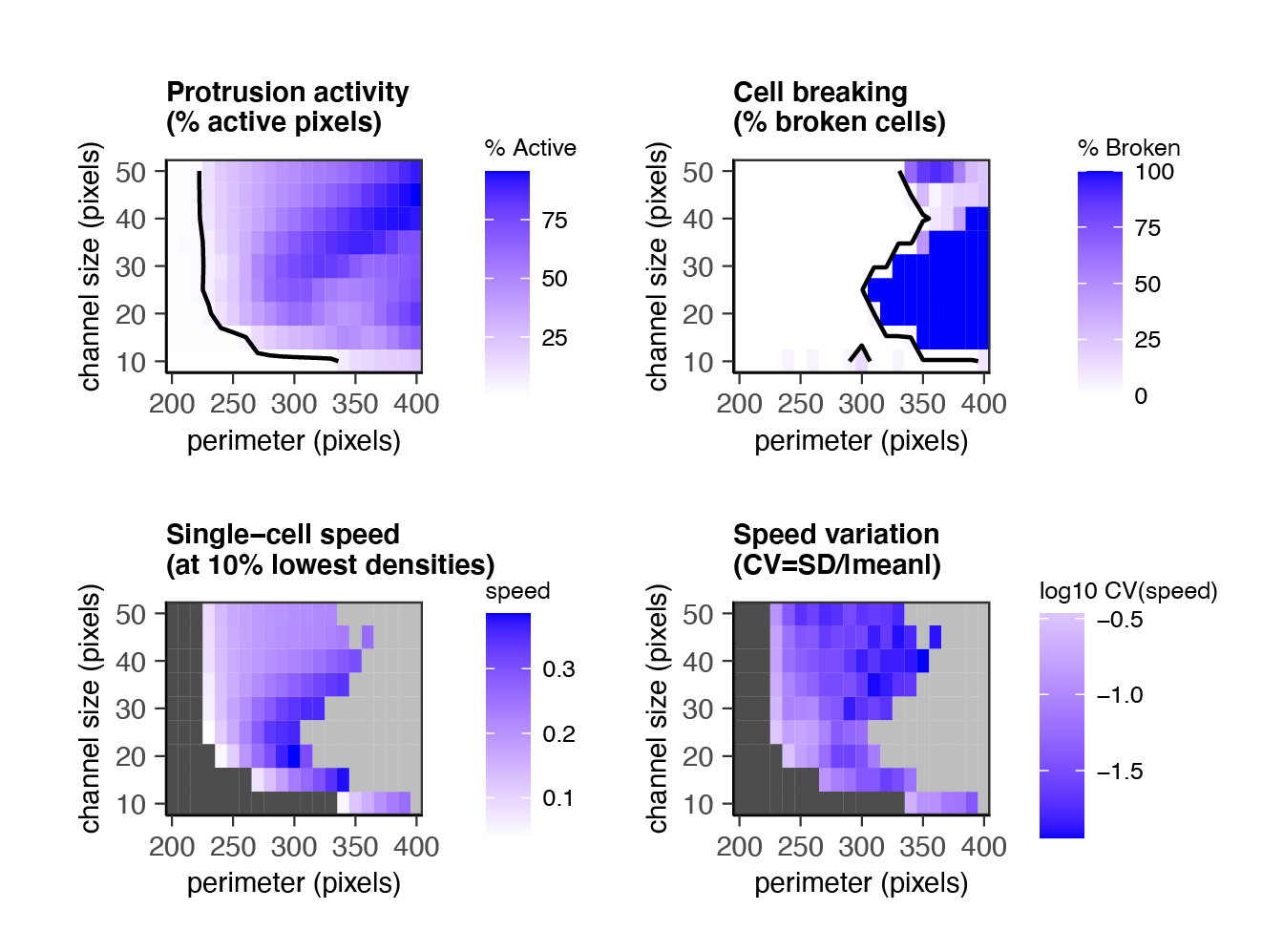
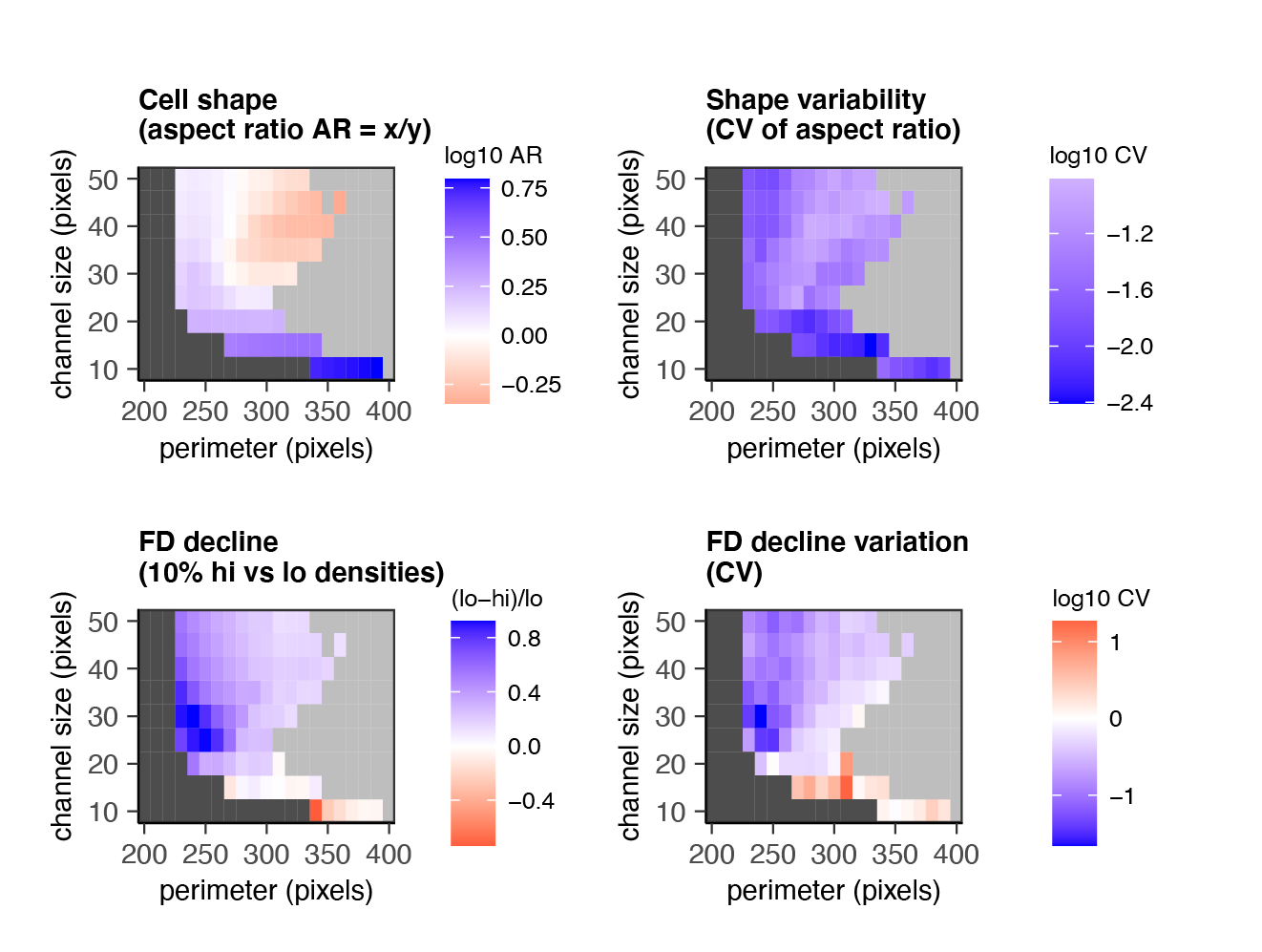

"What I cannot create, visualize, and take apart, I do not understand."
— Richard Feynman
Acknowledgments
 |
 |
 |
 |
 |
|
| Jérémy Postat | Connie Shen | Judith Mandl | Shabaz Sultan | Johannes Textor | |
| Mandl lab McGill University, Montréal, Canada |
Computational immunology group Radboud University, the Netherlands |
||||
| computational-immunology.org | |||||
 |
 |
||||